Translate this page into:
Epidemiology, treatment and prevention of herpes zoster: A comprehensive review
Correspondence Address:
Wu Jianbo
Associate Chief Physician, Department of Dermatology, Zhongnan Hospital of Wuhan University, Hubei - 430 071
China
| How to cite this article: Koshy E, Mengting L, Kumar H, Jianbo W. Epidemiology, treatment and prevention of herpes zoster: A comprehensive review. Indian J Dermatol Venereol Leprol 2018;84:251-262 |
Abstract
Herpes zoster is a major health burden that can affect individuals of any age. It is seen more commonly among individuals aged ≥50 years, those with immunocompromised status, and those on immunosuppressant drugs. It is caused by a reactivation of varicella zoster virus infection. Cell-mediated immunity plays a role in this reactivation. Fever, pain, and itch are common symptoms before the onset of rash. Post-herpetic neuralgia is the most common complication associated with herpes zoster. Risk factors and complications associated with herpes zoster depend on the age, immune status, and the time of initializing treatment. Routine vaccination for individuals over 60 years has shown considerable effect in terms of reducing the incidence of herpes zoster and post-herpetic neuralgia. Treatment with antiviral drugs and analgesics within 72 hours of rash onset has been shown to reduce severity and complications associated with herpes zoster and post-herpetic neuralgia. This study mainly focuses on herpes zoster using articles and reviews from PubMed, Embase, Cochrane library, and a manual search from Google Scholar. We cover the incidence of herpes zoster, gender distribution, seasonal and regional distribution of herpes zoster, incidence of herpes zoster among immunocompromised individuals, incidence of post-herpetic neuralgia following a zoster infection, complications, management, and prevention of herpes zoster and post-herpetic neuralgia.
Introduction
Herpes zoster, or shingles, occurs due to the reactivation of varicella zoster virus.[1] Adults above 50 years are at an increased risk for developing herpes zoster, probably due to the immunosenescence associated with advancing age, but it can affect individuals of any age, especially those with a suppressed cell-mediated immunity due to any disease or drugs.[2],[3] Complications due to the involvement of ophthalmic, splanchnic, cerebral, and motor nerves are reported in herpes zoster. However, the most commonly seen complication is post-herpetic neuralgia.[4] Vaccination against herpes zoster virus is the mainstay of prevention of herpes zoster infection.[5] Many treatment modalities have been developed for herpes zoster infection as well as for post-herpetic neuralgia. Nevertheless, approximately 22% of patients with herpes zoster still suffer from post-herpetic neuralgia.[6] Rise in the incidence of herpes zoster and post-herpetic neuralgia is expected with the increase in life expectancy and increase in prevalence of the modern-day epidemic human immunodeficiency virus (HIV). Wider use of varicella vaccination leads to reduced prevalence of varicella, thereby resulting in reduced chances of periodic re-exposure to varicella. This in turn can reduce natural boosting of immunity and lead to an increased incidence of herpes zoster.[7],[8] The main purpose of our study is to determine the incidence, risk, and complications of herpes zoster among healthy and immunocompromised patients and to improve the care of patients by accurate diagnosis, early management, and by methods to prevent herpes zoster and its recurrence.
Etiopathogenesis
Varicella zoster virus is one of the eight herpes viruses that are pathogenic only for humans.[9] It causes a primary infection called varicella/chicken pox, most commonly in children that is highly contagious.[10] It is most commonly transmitted by the airborne route from person to person or by direct contact with the lesion.[11] During the primary infection, the virus disseminates through the blood stream to the skin, oral mucosa, and lymph nodes, causing the generalized rash of varicella.[12]
After a primary infection or vaccination, the varicella zoster virus remains dormant in the sensory dorsal root ganglion cells. Resolution of the primary infection causes an induction of the varicella zoster virus-specific memory T cells. The memory T cell immunity declines over time. The decline below a theoretical “zoster threshold” correlates with an increased risk of herpes zoster infection.[10],[13] The memory immunity to varicella zoster virus may be enhanced by exogenous boosting (by exposure to varicella) or endogenous boosting (subclinical reactivation from latency).[10] The average period of immunity against varicella following an infection is 20 years.[14] Age, stress, immunocompromised status, and immunosuppressive drugs are known factors for virus reactivation.[15] It is recommended to determine the HIV status of those who develop herpes zoster.[16] Once the virus is reactivated, it travels along the affected sensory nerve, causes neuronal damage, reaches the respective dermatomes, and forms the vesicular rash of herpes zoster.[11] Herpes zoster infection is usually characterized by a unilateral, painful vesicular rash which is limited to a single dermatome.[17] Studies have shown that more than 95% of adults are infected with varicella zoster virus and therefore are at a risk of developing herpes zoster.[10] After an infection with herpes zoster, the chance of injury to the peripheral and central nervous system is high leading to post-herpetic neuralgia. The two main factors that play a role in the development of post-herpetic neuralgia are sensitization and deafferentiation.[18],[19],[20] The frequency of involvement is thoracic > lumbar and cervical > sacral. An increased spread of the herpes zoster virus beyond the isolated ganglion nerve dermatome unit is seen among patients who have a deficiency in T lymphocyte and macrophage-mediated immune defense. Involvement of lungs, central nervous system (CNS), mucous membranes, liver, cardiovascular system (CVS), bladder, skeletal system, blood vessels, and gastrointestinal system can be seen among patients with disseminated diseases. Involvement of the lungs, liver, and CNS can be fatal.[12]
Herpes zoster does not occur following exposure to varicella zoster virus.[21] However, herpes zoster affected individuals can transmit varicella zoster virus to seronegative contacts. These contacts develop varicella, not herpes zoster. Individuals exposed to herpes zoster are at a lower risk (16%) of developing varicella infection, when compared to those exposed to varicella zoster virus (61 − 100%).[7],[15] Transmission of varicella zoster virus from cases of herpes zoster occurs most commonly through direct contact with lesions than from airborne route.[7] Varicella zoster virus vaccination among children has shown to cause a long-term reduction of risk among vaccinated individuals in developing herpes zoster.[22] However, a study by Brisson et al. showed that a mass childhood immunization against varicella zoster virus caused an increase in the incidence of herpes zoster during the first 30 − 50 years of life.[14] The pathogenesis behind the reactivation of varicella zoster virus is unknown. But, any factor affecting the cell-mediated immunity may play a role in the reactivation of varicella zoster virus.
Epidemiology
Incidence
A systematic review published in 2014 reported an incidence of 3−5/1000-person years (PY) for herpes zoster in North America, Europe, and Asia- Pacific, with an increased incidence of 6 − 8/1000-person year at 60 years of age and 8 − 12/1000-person year at 80 years of age.[23] Three studies performed in Italy in the year 1999, 2004, and 2010 showed an incidence rate of 4.14/1000-person year, 1.59/1000-person year, and 6.31/1000-person year, respectively, showing that the incidence of herpes zoster varies from year to year.[24],[25],[26] The yearly variation of herpes zoster incidence in several countries has been listed in [Table - 1][24],[25],[26],[27],[28],[29],[30],[31],[32]. 10-20% of herpes zoster patients manifest herpes zoster ophthalmicus with a life-time risk of 1%.[33]

Age distribution
The incidence of herpes zoster increases with age which was proven by a population-based study conducted in Korea that reported an incidence of 2.0/1000-person year among the childhood group to 21.8/1000-person year in those aged 70−79 years. Peak incidence of herpes zoster is documented in the 60−69 age group and a low incidence is noted in those aged above 80 years.[34]
Legami et al. reported an increased incidence of hospitalization for herpes zoster among patients aged >72 years (0.46/1000-person year), compared to those aged 15−44 years (0.03/1000-person year),[26] suggesting that advancing age is a risk factor for herpes zoster requiring hospitalization. According to a US study with data from medstat marketscan, an increased number of hospital admissions, cost burden, complications, outpatient visit, and prescription for pain were noticed among older individuals than younger individuals.[35] Post-herpetic neuralgia, bacterial infections, ocular involvement, neurological involvement, and disseminated herpes zoster are documented as common complications warranting hospitalization in herpes zoster.[36] The average length of hospital stay for principal diagnosis of herpes zoster in patients aged 50 years and more was 6.8 days in a study by Stein et al. However, the same study showed an average hospital stay of 15.5 days for those with non-principal diagnosis [rehabilitation, chronic obstructive pulmonary disease (COPD), pneumonia etc.] of herpes zoster.[36] In a study comparing patients based on age at onset of herpes zoster ophthalmicus (<60 years and ≥60 years), Ghaznawi et al. observed a peak occurrence of herpes zoster ophthalmicus among individuals aged 50−59 years, which may be an effect of the mandatory childhood varicella vaccination leading to a higher incidence of herpes zoster infection among younger individuals.[37] Many have recommended vaccination against herpes zoster for individuals above 60 years, but some others have suggested that it is better to vaccinate immunocompetent patients <60 years also, considering the severity and chronicity of the disease in young adults as well.[37]
Gender distribution
Based on many studies, gender has a major role in herpes zoster incidence. Kim et al. showed that the incidence of herpes zoster was high for women when compared with men (12.6/1000-person year vs. 8.3/1000-person year).[34] Gauthier et al. and Gialloreti et al. also showed an incidence of 6.05/1000 and 4.75/1000-person year for women compared to men (4.30/1000 and 3.82/1000-person year).[24],[30] A retrospective cohort study done in China showed a female and male incidence of 3.95/1000-person year and 2.89/1000-person year, respectively.[38] According to these studies, the reason behind a higher female incidence can be a difference in the immune response to the latent virus infection.[24],[39] Some studies have shown no statistical difference in the incidence rate among male and female gender.[26],[40],[41],[42] While some studies show a male predominance, which has been supported by studies from India, Nepal, and Pakistan showing a male to female ratio of 1.74:1, 2.16:1, and 2:1, respectively.[43],[44],[45] The disparity noted in the sex distribution of herpes zoster in various studies could be due to difference in sample collection or a less chance for males to seek medical care compared to females, as shown by a shingles prevention study.[21] Our review suggest that herpes zoster is not a gender-specific disease. However, further research on gender distribution is required for determining any sex predilection for herpes zoster.
Seasonal and regional distribution
Seasonal variation for herpes zoster was noted in some studies showing a high chance of infection during the onset of summer.[44],[45] However, other studies documented no significant seasonal variation for herpes zoster and post-herpetic neuralgia.[34],[46] An increased incidence of herpes zoster was shown in the urban region (7.65/1000-person year) when compared to rural area (2.06/1000-person year) in a study conducted in China.[38] Similarly, an increased degree of urbanization when compared with municipalities was noted in a study from Netherlands.[31] However, there are still other studies that do not show any difference in incidence between the rural and urban areas.[47],[48] This shows that more studies are required to confirm the seasonal and regional variations in incidence of herpes zoster.
Incidence in immunocompromised individuals
Several previous studies have shown that herpes zoster incidence is high among immunocompromised individuals when compared with healthy population. A study by Chen et al. observed higher incidence of herpes zoster among bone marrow or stem cell transplant recipients (43.03/1000-person year) than among solid organ transplant recipients (17.04/1000-person year).[49] HIV, systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), cancers, inflammatory bowel disease (IBD), multiple sclerosis, and psoriasis place a patient at higher risk for herpes zoster.[49] An earlier study from San Francisco reported herpes zoster incidence of 29.4/1000-person year among HIV seropositive individuals compared to HIV seronegative individuals (2.0/1000-person year) and other controls. Cellular immunity plays a role in the inactivation of the herpes zoster virus. Patients with HIV experience a decline in their CD4+ cells and an increase in their CD8+ cells, leading to an increase in the incidence of herpes zoster infection.[50] Insinga et al. reported an incidence of 10.3/1000-person year for herpes zoster among patients in all age groups with cancer, HIV infection, or transplantation compared to general population (3.0/1000-person year).[27] Another study carried out in cancer patients by Yenikomshian et al. showed that the incidence of herpes zoster was low for individuals affected with prostate cancer (12.3/1000-person year) and high for those with Hodgkin's lymphoma (47.8/1000-person year). The same study reported a higher incidence among hematologic cancers (31.0/1000-person year) compared with solid organ cancers (14.9/1000-person year).[51] Moreover, a higher incidence was noticed among those receiving immunosuppressants or chemotherapy agents.[49] Although those receiving immunosuppressive medications are at a high risk for developing herpes zoster, a study by Megna et al. reported that biologics for the treatment of psoriasis was not associated with any significant increase in the risk for developing the same.[52] Two different studies from Japan and Israel showed an increased incidence of herpes zoster infection in patients with diabetes.[53],[54] Herpes zoster is also seen more frequently among patients with COPD, hypertension (HTN), Sjogren's syndrome, psychiatric diseases, osteoskeletal diseases, ocular diseases, and renal failure.[53],[55],[56],[57]
Risk of post herpetic neuralgia
The incidence of post-herpetic neuralgia following herpes zoster is high. Gauthier et al. reported that 19.5% of herpes zoster patients develop PHN1 (pain persisting at least 1 month after rash onset) and 13.7% develop PHN3 (pain persisting at least 3 months after rash onset).[30] Similarly, an Italian study showed a proportion of 9.4% for PHN1 and 7.2% for PHN3 among immunocompetent patients with herpes zoster.[24] The incidence of post-herpetic neuralgia increases with age and this observation was supported by Stein et al. They showed an increased incidence of post-herpetic neuralgia of 3.16/1000-person year for those aged ≥80 years compared to those aged 50−59 years (0.73/1000-person year) and those younger than 50 years (0.08/1000-person year).[36] Post-herpetic neuralgia has a tendency to affect women more than men.[24],[30],[46] Post-herpetic neuralgia tends to have a longer duration when compared with herpes zoster infection, and thus the pain burden affects the quality of patient's life.[58] Studies reporting the risk of post-herpetic neuralgia following herpes zoster have been listed in [Table - 2].[24],[25],[30],[32],[40],[41],[59],[60],[61],[62]

Clinical Features
Herpes zoster infection usually begins with prodromal symptoms such as pain, fever, malaise, headache, itch, and paresthesias which precede the rash by a few hours to several days in most patients.[12],[18] Due to the frequent itching or pain that develops before the appearance of the rash (zoster sine herpete), there can be a chance of delayed diagnosis.[15] Following the prodromal phase, the active phase begins when the patients manifest the characteristic skin lesions such as erythematous papules or macules which progress to vesicles in 12−24 hours, to pustules in 1−7 days, and eventually crust over in 14−21 days (resolution phase).[12],[63] The chronic phase of the disease is associated with the development of post-herpetic neuralgia, involvement of cranial nerves, and involvement of visceral organs.[12]
Post-herpetic neuralgia is defined as a herpes zoster pain persisting for more than 3−6 months after the onset of the rash, or pain lasting even after complete healing of the rash.[36] In most of the patients, post-herpetic neuralgia is characterized by severe, constant, or intermittent burning or lancinating pain with allodynia.[16]
Involvement of the fifth cranial nerve (trigeminal) leads to Ramsay Hunt syndrome and herpes zoster ophthalmicus. Severe otalgia and erythematous vesicular rash in the external auditory canal and pinna manifest as herpes zoster oticus. When associated with ipsilateral facial palsy it is known as Ramsay Hunt syndrome. The facial and auditory nerves are infected by varicella zoster virus leading to herpetic inflammation of the geniculate ganglion [64] The characteristic symptom of herpes zoster oticus includes pain in and around the ear followed by vertigo, hearing loss, tinnitus, nausea, and vomiting.[65] Most patients with vertigo experience hearing loss. However, patients without vertigo do not develop hearing loss.[64],[66] Though a rare manifestation of herpes zoster in childhood, Ramsay Hunt syndrome is known to be the second most common cause of facial paralysis after Bell's palsy among children.[67]
Reactivation of the virus in the ophthalmic (V1) division of the trigeminal nerve results in herpes zoster ophthalmicus. If the vesicles are present on the side and tip of the nose (Hutchinson's sign), the external division of the nasociliary branch is affected indicating the probability of involvement of eye (in approximately 76% cases). If vesicles are present on the lid margins, it indicates ocular involvement.[33] The complications associated with herpes zoster ophthalmicus initially affect the skin and anterior segments of the eye, later involving the optic nerve, retina, and CNS. The risk and complication associated with herpes zoster ophthalmicus is more common in the elderly and immunocompromised patients.[33],[68]
Complications
Complications of herpes zoster are more common among elderly individuals and immunosuppressed patients. Herpes zoster and its complications can impact the patient's quality of life. In most patients, sleep and social activities are affected. Post-herpetic neuralgia is the most common complication of herpes zoster. The other complications noted following post-herpetic neuralgia, include secondary bacterial infections, ophthalmic complications, cranial and peripheral nerve palsies, and segmental zoster paresis.
Severe post-herpetic neuralgia can lead to sleep disturbance, depression, weight loss, chronic fatigue, and inability to perform daily activities. The pain may extend beyond the involved dermatome.[69] The severity of post-herpetic neuralgia is usually dependent on the presence of pain prior to rash formation, rash severity, inflammation, older age, and immunocompromised status. The treatment of post-herpetic neuralgia is often challenging due to lack of definitive treatment algorithm for the condition.[15],[69]
Secondary bacterial infection such as cellulitis, septicemia, zoster gangrenosum, and necrotizing fasciitis caused by Staphylococcus aureus and Streptococcus pyogenes are the most common complications seen after post-herpetic neuralgia. Elderly and immunocompromised patients are more prone to bacterial infections.[70],[71] Cellulitis can lead to necrosis and scarring.[72] Necrotizing fasciitis is a serious condition which can be complicated by a streptococcal toxic shock-like syndrome.[73]
A rare, but serious complication of herpes zoster ophthalmicus is granulomatous arteritis. The condition is characterized by headache and hemiplegia on the contralateral side of the lesion secondary to stroke.[70] Other complications associated with herpes zoster ophthalmicus include blepharitis, conjunctivitis, epithelial keratitis, stromal keratitis, neurotrophic keratopathy, uveitis, episcleritis, scleritis, acute retinal necrosis (ARN, and progressive outer retinal necrosis syndrome (PORN).[74] Acute retinal necrosis and progressive outer retinal necrosis syndrome are two herpes zoster ophthalmicus complications that lead to retinal detachment. Compared to acute retinal necrosis, PORN syndrome is more severe with a poor prognosis and is more commonly seen in patients with advanced AIDS or in patients with other disease conditions causing immunosuppression.[74],[75] According to a report by Tran et al., the recurrence rate of herpes zoster ophthalmicus complications at 1, 3, 5, and 6 years were 8%, 17%, 25%, and 31%, respectively.[76] This proves that ocular complications can sometimes recur after a long period of up to 10 years following a zoster episode.
Herpes zoster myelitis (HZM) is a rare neurologic complication with acute onset affecting most commonly patients with immunocompromised status. It occurs shortly after the onset of rash with development of sensory, motor, and autonomic dysfunction.[77] Ong et al. reported that neurological progression caused by herpes zoster myelitis can be prevented with oral antiviral therapy even after a delay in diagnosis.[77]
Segmental zoster paresis is a neurologic complication following herpes zoster infection and is characterized by focal, asymmetric motor weakness affecting the myotome corresponding to the dermatome distribution of the rash.[78] In case of segmented zoster abdominal paresis, it can lead to abdominal wall weakness and abdominal wall pseudohernia. It most commonly affects the middle aged and elderly individuals.[79] The abdominal weakness often leads to a flank bulge which can be misdiagnosed as an abdominal wall hernia. The condition is usually self-limiting with a good prognosis.[79],[80] Teo et al. reported a case of segmented zoster paresis involving the lower limb in a patient with multidermatomal herpes zoster infection, with a good prognosis and complete recovery of limb weakness with complete resolution of rash.[81] Involvement of the facial nerve can lead to Bell's palsy.[79] Segmented zoster paresis involves the thoracic dermatome in half of the cases, followed by facial, cervical, and lumbosacral dermatomes. Nevertheless, a high frequency of motor complications is seen with facial and limb involvement.[78]
Acute urinary retention is a complication observed in elderly and young immunocompromised patients with herpes zoster due to involvement of the spinal cord, spinal sensory ganglia, or sacral nerve roots.[82] Patients with sacral herpes zoster have higher chances of developing urinary or bowel dysfunction.[83] In most cases, patients experience bladder dysfunction as soon as the rash develops or within few days of rash onset.[82] Rothrock et al. reported a case of herpes zoster with neurogenic bladder where the rash appearance was delayed until six weeks after the initial onset of urinary retention.[82]
Diagnosis
The diagnosis of herpes zoster can be made clinically once the rash appears. Tzanck smear and electron microscope (EM) can detect the presence of a herpes virus from the vesicle. However, it cannot distinguish between herpes simplex virus and varicella zoster virus.[7] Polymerase chain reaction (PCR), direct immunofluorescence assay (DFA), skin biopsy, and viral culture are the laboratory diagnostic tests of atypical herpes zoster.[18] PCR can detect varicella zoster virus DNA in the vesicular fluid, and hence, is considered the most sensitive and specific diagnostic test for herpes zoster. PCR can be done on fluid from lesion, blood, plasma, cerebrospinal fluid (CSF), and bronchoalveolar lavage.[63] DFA can be used as an alternative to PCR. It is preferred over viral culture due to its high sensitivity, low cost, and turnaround time compared to viral culture.[84] In patients with herpes zoster myelitis, viral isolation cannot be done from blood or CSF fluid. Hence, diagnosis of herpes zoster myelitis can only be made by clinical appearance of rash on the particular dermatome with clinical features of transverse myelitis and magnetic resonance imaging (MRI) of the spine.[77] In case of segmental zoster paresis, diagnosis can be confirmed by the presentation of painful dermatomal rash with muscle weakness. An electromyography can reveal acute denervation of the compromised area.[78]
Treatment
The main aims of treatment for herpes zoster are to decrease pain, induce quick healing, and avoid complications. Antiviral therapy is used for the treatment of herpes zoster as soon as a diagnosis is made, and it reduces the risk of post-herpetic neuralgia. Corticosteroids can help to control pain and eruptions. Other components of therapy include isolation of patient and local management of skin lesions. Isolation of patient is necessary to prevent nosocomial infections.
Antiviral agent
Antivirals such as acyclovir, famciclovir, and valacyclovir are used to reduce acute herpes zoster. These agents help in reducing pain, promote fast healing, and prevent post-herpetic neuralgia. Treatment with antiviral should be started within 72 hours of rash onset.[18] Famciclovir is shown to be superior to valacyclovir in reducing acute herpes zoster pain, which was supported by a Japanese study by Ono et al. They observed an earlier reduction in pain within 3−4 days in a 7-day treatment course with famcyclovir.[4] A retrospective study by Lam et al.showed that oral acyclovir and valacyclovir are not associated with a higher risk of acute kidney injury when compared with famciclovir. The use of intravenous (IV) acyclovir is associated with acute kidney injury. The drug results in precipitation and crystallization in the tubules causing obstruction and cellular necrosis.[85] Hence, IV acyclovir is an absolute contraindication for patients with renal disease. A short course of acyclovir therapy (800 mg five times a day for 4 days) showed a similar efficacy for patients presenting with rash duration for >72 hours and for duration <72 hours.[86]
Acyclovir plus ultraviolet B (UVB) have been shown to reduce the incidence of subacute herpetic neuralgia (41.67%) and PHN (16.67%) compared to those treated with acyclovir alone (61.54% and 46.15%). Adverse effects such as erythema and first-degree burn were seen among patients receiving ultraviolet B. However, the patients recovered after decreasing the dose.[87]
Oral acyclovir given within 72 hours of onset of rash can reduce the incidence and severity of herpes zoster ophthalmicus by reducing the pain and other long-term complications.[88],[89] A study by Aylward et al. showed no beneficial effect of oral acyclovir on ocular complications of herpes zoster ophthalmicus. This may be due to a late onset of initial treatment.[90] Topical acyclovir has no prophylactic effect in managing herpes zoster ophthalmicus.[91] An 830 nm light-emitting diode (LED) therapy + famciclovir showed faster wound healing and decreased pain score among herpes zoster ophthalmicus patients compared to famciclovir alone.[92] Visual impairment and blindness are often noticed with herpes zoster ophthalmicus complications. Hence, the necessity of early management of herpes zoster ophthalmicus with antiviral in a primary health care center is required before referring the patient to a higher ophthalmology center.[68] In a survey conducted among cornea specialists and ophthalmologists on their opinion on management of recurrent and chronic herpes zoster ophthalmicus, 56% of respondents preferred acyclovir prophylaxis for preventing or reducing herpes zoster ophthalmicus incidence, 63% preferred oral antiviral + topical steroid for patients with signs of recurrent disease, and 64% of respondents did not believe in the adult zoster vaccine for reducing the recurrence rate of herpes zoster ophthalmicus.[93] Ganciclovir gel has shown a rapid healing effect in patients with persistent or recurrent pseudodendrites in herpes zoster ophthalmicus. The drug acts only on the infected cells and hence has a good efficacy and is less toxic.[94] Intravenous acyclovir 1500 mg/m2/day divided into three doses for 7−10 days followed by oral acyclovir 800 mg five times a day for 14 weeks is recommended for acute retinal necrosis/PORN syndrome.[74]
Systemic corticosteroids
Corticosteroid therapy is recommended for special situations such as acute zoster pain, Ramsay Hunt syndrome, and ocular complications. Corticosteroid therapy is more beneficial when combined with an antiviral agent. Early administration of acyclovir + steroid has shown good improvement among adults and children in the treatment of herpes zoster oticus/Ramsay Hunt syndrome.[67],[95],[96],[97],[98] Hearing recovery also increases with early therapy.[98] The prognosis of Ramsay Hunt syndrome is good for patients with an early age onset and those who receive combined antiviral + steroid treatment within 72 hours of rash onset.[99] Combination therapy with acyclovir and steroid for Ramsay Hunt syndrome has shown improvement in facial nerve function.[65],[96],[98] Murakami et al. showed efficacy of acyclovir and steroid combination for managing Ramsay Hunt syndrome with acyclovir 250 mg IV three times daily/oral acyclovir 800 mg five times daily for 7 days along with IV/oral prednisone 1 mg/kg/day two times a day for 5 days, tapered to zero over the following 10 days.[98] Coulson et al. managed patients with Ramsay Hunt syndrome with oral acyclovir 200 mg 5 times a day for 21 days along with oral prednisone 1 mg/kg for 14 days tapered10 mg/day to zero.[65] The dosage, route of administration, and period of treatment have varied in different studies.[97] Combined acyclovir and prednisolone treatment for herpes zoster in patients aged more than 50 years have shown to improve quality of life.[100] Acyclovir + prednisolone can clear rash and reduce acute illness with herpes zoster, however a long-term effect in the prevention of post-herpetic neuralgia is not known.[101] A study comparing ACTH and prednisone reported that neither of the two agents were effective in preventing post-herpetic neuralgia.[102] Two early studies by Eaglstein et al. and Elliot et al. have shown reduction of post-herpetic neuralgia with early oral corticosteroid therapy. However, both studies had the limitation of small sample size.[103],[104] Intravenous acyclovir 10−15 mg/kg three times daily for 7 days and prednisolone 60−80 mg three times daily for 5 days is indicated for the management of granulomatous arteritis, but a risk of irreversible cerebral infarction is often observed by the time of diagnosis.[70] The efficacy of corticosteroids in managing herpes zoster and preventing post-herpetic neuralgia remains unclear.
Acyclovir resistant herpes zoster
Acyclovir resistance is commonly observed among severely immunocompromised patients receiving long-term acyclovir therapy for varicella zoster virus and herpes zoster virus infection.[105],[106] Acyclovir resistance occurs due to a mutation in the viral thymidine kinase that suppresses the enzymatic activity.[105] A study from Turkey reported that early recognition of resistance and the use of foscarnet and cidofovir can reduce mortality among immunocompromised patients.[106] The recommended dose of foscarnet for acyclovir resistant varicella zoster virus infection is 120 mg/kg/day (40 mg/kg thrice a day or 60 mg/kg twice a day).[107] Breton et al. adopted a higher dose of foscarnet (200 mg/kg/day) for acyclovir resistant herpes zoster in their study, and 10 of 13 patients responded well to the therapy.[107] The study suggested an increase in the dose of foscarnet from 120 to 200 mg/kg/day for acyclovir-resistant herpes zoster.[107] The mutation in the viral DNA polymerase sometimes cause an inability to identify acyclovir triphosphate leading to a cross-resistance to foscarnet.[106] A study by Blot et al. reported that cidofovir can be used as a salvage therapy for patients with severe herpes simplex virus (HSV) infection who are resistant to acyclovir and foscarnet.[108] Cidofovir is a monophosphate nucleotide analogue which is converted to its active form cidofovir diphosphate independently without any viral participation. Hence, viral mutation causing an altered phosphorylase activity does not lead to cidofovir resistance.[108],[109] Ross et al. suggested the use of combined therapy with intralesional interferon alfa-2b and 1% trifluorothymidine ophthalmic solution as a third-line therapy for acyclovir resistant herpes zoster when other treatments fail.[110]
Treatment of Herpes Zoster in Pregnancy
Acyclovir or valacyclovir can be used for the treatment of herpes zoster in pregnancy.[111] Acyclovir is considered as the drug of choice (DOC) in early pregnancy with no increased risk of malformation or preterm births.[112] A 28-week primigravida treated with acyclovir + acetaminophen for herpes zoster neuralgia responded well to the drugs and 2 months later delivered a healthy baby.[113] A 17-week pregnant woman treated with valacyclovir for herpes zoster responded well to the drug.[114] According to the prevention of varicella recommendations by centers for disease control and prevention advisory committee on immunization practices (CDC ACIP), varicella zoster immune globulin is strongly indicated for susceptible pregnant women exposed to varicella to prevent varicella-related complications in pregnancy as well as to immunocompromised children for passive immunization after substantial exposure to varicella zoster virus or herpes zoster virus.[115] Neonates whose mothers have varicella infection or herpes zoster within 5 days before and 2 days after delivery are advised to receive varicella zoster immune globulin regardless of the maternal history of varicella zoster immune globulin administration. varicella zoster immune globulin is not necessary to be given to healthy neonates whose mothers had varicella for more than 5 days prior to delivery since the child is already protected from varicella by transplacental acquired maternal antibody.[115] Premature infants exposed postnatally to varicella or herpes zoster are recommended to be given varicella zoster immune globulin due to their weakened immune system and less chance of acquiring transplacental maternal antibody.[115]
Treatment of Herpes Zoster in Children
Herpes zoster is rare in children, and if occurs, is usually benign. If the child continues to develop new lesions even after 3 weeks of infection, an underlying immunodeficiency should be considered.[116] Oral suspension of acyclovir can be used in children. However, it is not routinely used for the treatment in preadolescent children since it is not approved for those age groups.[116] Treatment with acyclovir is recommended for preadolescent children if they have an ocular involvement, immunocompromised status, or malignancies.[117],[118] Otherwise no specific treatment is given for this age group.[116],[119] Four infants aged 4−11 months with infantile herpes zoster were treated with oral (three cases) and IV (one case) acyclovir and recovered completely without sequelae. All four infants had a prior history of exposure to varicella zoster virus.[120] Zoster in infants and neonates can be due to a maternal varicella zoster virus infection during pregnancy.[116] Children experience herpes zoster in the first decade of life due to varicella zoster virus infection before 2 months of age.[116] Similarly, children in the first two decades of life may develop herpes zoster due to infection with varicella zoster virus before 12 months of age.[116] According to the prevention of varicella recommendation by CDC ACIP, varicella zoster vaccine is recommended only for children aged ≥1 year and the vaccine is not indicated for preventing zoster.[115] Similarly, herpes zoster prevention by CDC ACIP does not recommend herpes zoster vaccine for any patient <60 years of age.[121] There is no vaccine recommendation for children to prevent zoster infection after an exposure to varicella during the first year of life. Neonatal herpes zoster is very uncommon with an estimated incidence of 0.74 cases/annum. But, if it occurs, it is usually left untreated due to its benign nature in the preadolescent group unless a severe infection or ocular involvement caused by zoster are noticed.[119]
Treatment of Herpes Zoster in Immunocompromised Individuals
Acyclovir has a known prophylactic action for the prevention of herpes zoster among immunocompromised patients.[122],[123],[124],[125] A study in Africa among HIV-infected patients with CD4+ T cell count >250 cells/μL showed that acyclovir prophylaxis (400 mg twice daily) reduced the chance of herpes zoster by 62%.[126] Localized herpes zoster in immunocompromised patients was well treated with topical acyclovir. The ointment was applied four times a day for 10 days. Topical therapy can help reduce the period of hospitalization required for IV drug administration and reduces the side effects associated with IV treatment.[127] Oral brivudin is as effective as IV acyclovir for herpes zoster among patients with underlying malignant diseases. A 5-day oral dosage of 125 mg every 6 hours is recommended and can be given on an outpatient basis.[128] Outpatient therapy with valacyclovir 1-2 g three times daily was shown to be cost-effective for herpes zoster in immunocompromised patients compared to those treated with IV acyclovir as inpatients.[129] In a study by Tyring et al. on 149 immunocompromised patients comparing famciclovir and acyclovir for herpes zoster, famciclovir was well tolerated and was recommended as an alternative for acyclovir.[130] An HIV patient treated with valacyclovir did not respond well to the drug. However, later the patient showed favorable outcome with IV acyclovir.[124] Further large-sized clinical trials are required to study the efficacy of valacyclovir. In patients taking bortezomib for multiple myeloma, acyclovir has been used has a prophylactic agent to prevent herpes zoster.[125],[131] Summary of treatment for herpes zoster is described in [Table - 3].
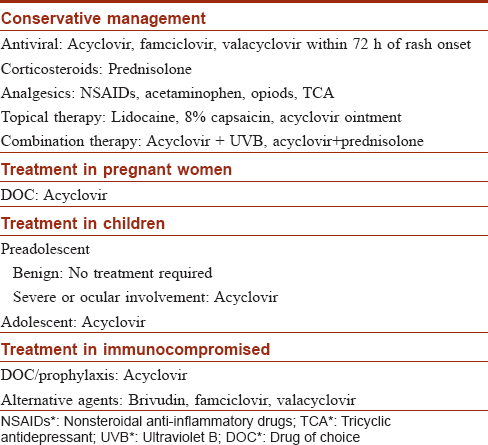
Management of Post-Herpetic Neuralgia
Antivirals are the main stay of treatment for herpes zoster and for reducing the risk of post-herpetic neuralgia; however, it does not prevent post-herpetic neuralgia.[1] Several analgesics such as acetaminophen, NSAIDs, opioids, tricyclic antidepressants (TCA), anticonvulsants, and topical agents are used along with antivirals for reducing the pain associated with herpes zoster.
Topical Therapy
Topical capsaicin 8% patch is beneficial in managing trigeminal post-herpetic neuralgia. Pain reduction mechanism with capsaicin is unknown but it is thought to be due to the reduction in substancePin the skin.[132] The major adverse effect with topical capsaicin cream is a burning sensation on the applied site. It has to be applied three to five times a day.[12] Topical 5% lidocaine plasters (≤3 patches/day for 12 hours/day) have shown great benefit for patients with post-herpetic neuralgia especially among the elderly individuals due to its decreased side effects compared to other systemic agents.[133],[134] Lidocaine-medicated plasters relieve pain by the action of absorbed lidocaine on sodium channels of sensitized afferents in the affected skin, and through the barrier effect which protects the allodynic skin from mechanical stimuli.[133]
Pharmacological Therapy
Earlier, tricyclic antidepressants (TCA) were used as the first-line in the treatment of post-herpetic neuralgia. However, later due to its increased side effects, including anticholinergic action, gabapentin was preferred over tricyclic antidepressants.[135] Carbamazepine, a first generation anticonvulsant, is effective in managing chronic neuropathic pain, but several cases of carbamazepine-induced Stevens − Johnson syndrome and toxic epidermal necrolysis have been reported, and hence, it is not recommended.[136] Gabapentin has shown good effect on sleep and quality of life for the patient.[137] A once daily dose of gastroretentive gabapentin (G-GR) of 600 mg reported rapid pain reduction on day 2 with a decreased incidence of adverse effects.[138] Pregabalin is often recommended as the first line in the treatment of post-herpetic neuralgia, but at an increased cost.[139] However, a study by Pérez et al. showed no significant cost differences between gabapentin and pregabalin.[140] Gabapentin acts by binding to the α2δ-1 subunit of voltage-gated calcium ion channel by reducing their action on dorsal root ganglion (DRG) by inhibiting membrane trafficking (cytoplasm to plasma membrane) and anterograde trafficking (axoplasmic transport). Gabapentin also shows acute analgesic effects by lowering the release of neurotransmitters such as substance P.[135] Gabapentin and pregabalin should be used with caution in patients with renal insufficiency.[141]
Combination therapy of pregabalin and oxycodone has shown a decrease in pain intensity and an improvement in quality of life.[142] Another study comparing the efficacy of amitriptyline 25 mg OD and pregabalin 75 mg BD showed better efficacy in the pregabalin group in the treatment of post herpetic neuralgia, with good improvement at the end of 8th week.[143] A 4- week study by Xu et al. reported that cobalamin is effective for managing the pain and discomfort in sub-acute herpetic neuralgia (SHN) patients. Local SC injection of MeB12 is more safe and effective for elderly patients than systemic therapy.[144] Intravenous use of vitamin C has also shown to be effective for managing herpes zoster associated pain.[145] Early diagnosis and treatment of herpes zoster can reduce the duration of herpes zoster and risk of post-herpetic neuralgia.
Interventional Therapy
Intrathecal and epidural injections
The potent anti-inflammatory action of corticosteroids may reduce the nerve damage, thereby preventing the pain of post-herpetic neuralgia.[146] An RCT comparing intrathecal midazolam and epidural methylprednisolone showed a prolonged analgesic effect on post-herpetic neuralgia of the lumbosacral dermatome. This effect can be due to the anti-nociceptive action of these agents on the spinal nerve roots.[147] A study on 598 patients more than 50 years of age with acute herpes zoster reported that a single epidural injection of methylprednisolone and bupivacaine reduces acute zoster associated pain for 1 month. The study did not show a long-term prevention of post-herpetic neuralgia with these agents.[148] According to Pasqualucci et al., epidural administration of methylprednisolone and a local anesthetic (bupivacaine) prevents post-herpetic neuralgia at 12 months, by only 1.6% patients reporting pain in the epidural analgesic + steroid group and 22.2% in acyclovir + steroid group.[149] However, a meta-analysis comparing 5 clinical trials reported that corticosteroids are useful for relieving the zoster associated pain during the acute phase of infection and has no effect in preventing post-herpetic neuralgia.[146] Epidural anesthetics and steroids have shown to reduce acute zoster associated pain, but more RCTs are needed for a clear understanding the effect of corticosteroids in the prevention of post-herpetic neuralgia.
Cryoanalgesics/Cryotherapy of the intercostal nerves
Cryotherapy of the intercostal nerve is an old technique, but is still in clinical practice for the management of severe thoracic pain caused by thoracotomy, rib fractures, post-thoracotomy syndrome, intercostal neuralgia, and post-herpetic neuralgia.[150] Cryotherapy causes pain relief by freezing the intercostal nerves at -60° C with carbon dioxide or nitrous oxide for 30 − 45 s using a cryoprobe leading to destruction of the myelin sheath and thereby blocking the nerve conduction.[151],[152] The nerve axon is left unharmed, so after the regeneration of the myelin sheath, the functional recovery of the nerves take place.[152] In a retrospective study conducted among 70 patients with chronic intercostal pain, the result for those patients suffering from post-herpetic neuralgia was poor. The author did not prefer cryotherapy of intercostal nerve for post-herpetic neuralgia.[153] Calandria et al. reported a new nonfreezing technique (NFT) by the application of liquid nitrogen (LN) spray into the skin of the affected dermatome forming a nitrogen cloud without allowing the area to freeze. Seventy-five percent of the patients showed excellent result. This technique was accepted as it did not cause freezing of the skin or did not lead to erythema or burning on the site of application.[154] The long-term reduction of chronic thoracic pain caused by post-herpetic neuralgia with cryoanalgesia is unclear. More RCTs on cryoanalgesia for the management of post-herpetic neuralgia are required for better understanding.
Spinal cord stimulation
Spinal cord stimulation device stimulates the sensitized dorsal horn neurons by restoring the impaired excitatory and inhibitory functions. The technique works only if there is no complete deafferentation or intraspinal neuronal death due to post-herpetic neuralgia.[155] A cure rate of 27−82% has been reported with spinal cord stimulation with an increasing cost of 52,091 USD per patients over a period of 24 months.[156] Several studies have shown that spinal cord stimulation is a worthwhile option for the management of post-herpetic neuralgia.[155],[157],[158] Temporary spinal cord stimulation has also been beneficial in reducing subacute herpetic pain and preventing its progression to chronic herpetic pain.[159] Another study from Japan showed a good effect of temporary spinal cord stimulation in reducing pain in the persistent phase after herpes zoster and preventing the transition to post-herpetic neuralgia.[160] Temporary spinal cord stimulation is less expensive and less invasive than conventional spinal cord stimulation. It is more convenient for use due to its lack of restriction with MRI.[161] Microsurgical dorsal root entry zone lesioning (DREZotomy) assisted with spinal cord stimulation is also an effective method for post-herpetic neuralgia.[156] Spinal cord stimulation given to chronic kidney disease (CKD) patients with post-herpetic neuralgia also helped in managing pain.[157]
Other interventional therapies
Deep brain stimulation of the contralateral periventricular grey area (PVG) and ventral posterior lateral thalamic nucleus (VPL) has been effective for controlling post herpetic neuralgia in a 30-year-old patient with a 10-year history of right-sided facial dysesthesia due to herpes zoster infection at 20 years age. The pain score at last follow-up at 6 months was 0/10.[162] More studies are required to confirm the efficacy of thalamic stimulation in post-herpetic neuralgia. According to Kolšek et al., transcutaneous electrical nerve stimulation provided pain relief and resolution of skin lesions with minimal complications of herpes zoster compared to antiviral agents and was considered as a good adjunct or even an alternative therapy to antivirals in the treatment of acute herpes zoster and in reducing post-herpetic neuralgia incidence.[163] Transcutaneous electrical nerve stimulation + local methylcobalamin has also shown good analgesic effect on post-herpetic neuralgia.[164]
Prevention
Zostavax, a live attenuated varicella zoster virus-based zoster vaccine, has shown to reduce the incidence of herpes zoster and post-herpetic neuralgia among immunocompetent individuals of ≥60 years, worldwide. The vaccine boosts the varicella zoster virus-specific cell-mediated immunity, thereby controlling the reactivation or replication of the latent varicella zoster virus and prevents herpes zoster infection or reduce its severity.[17] Both varicella vaccine and herpes vaccine are derived from the Oka varicella zoster virus strain. However, herpes vaccine has a 14-fold higher vaccine virus potency than varicella vaccine.[165] This live attenuated vaccine is not recommended for pregnant women, children, and immunocompromised patients.[165] Individuals with a prior history of herpes zoster can be vaccinated with herpes vaccine to prevent further episodes.[121] Administration of herpes vaccine in patients receiving biologic agents such as adalimumab, infliximab, and etanercept is contraindicated. However, CDC ACIP allows administration of herpes vaccine in these patients either 14 days before initiation of immunosuppressive therapy or one month after discontinuation of these agents.[121] The vaccine should not be administered if the patient is on antiviral therapy because the antiviral agent can prevent the replication of the vaccine virus leading to vaccine failure. Hence, patients on chronic antiviral therapy must stop medication at least 24 hours prior to vaccination and should not take the medicine for 14 days after vaccination.[121] The Canadian national advisory committee on immunization reported that patients taking low-dose immunosuppressive therapy can receive herpes zoster vaccination after an opinion from an immunodeficiency specialist depending on patient's case.[15] Even though any age group can develop herpes zoster infection, a vaccination recommendation has to be given for at-risk individuals, such as elderly people. In a randomized placebo controlled study by Lal et al., herpes zoster subunit (HZ/Su) vaccine reduced the risk of herpes zoster among individuals aged ≥50 years showing an overall vaccine efficacy of 97.2%. However, the study reported few vaccine-related adverse events in 4 participants.[2] A study by Domingo et al.showed intramuscular(IM) administration of Zostavax was well tolerated and had a similar immune response to that of subcutaneous (SC) administration. However, fewer injection site reactions and adverse effects were noted among intramuscular group than subcutaneous group. The preferred route of vaccine administration in some European countries is intramuscular. The administration route differs between countries and health care systems.[17] A large scale shingle prevention study by Oxman and Levin.reported a reduced incidence of herpes zoster burden of illness (BOI) by 61.1% after herpes zoster vaccine usage. Similarly, the incidence of herpes zoster and post-herpetic neuralgia was also reduced by 51.3% and 66.5%, respectively. The study showed that herpes zoster vaccine reduced the morbidity rate among elderly adults with herpes zoster and post-herpetic neuralgia. Only mild injection site reactions were noted.[166] Routine vaccination is recommended for all patients above 60 years excluding patients who are severely immunocompromised and allergic to any vaccine forms.[121] Because the treatment cost of herpes zoster and post-herpetic neuralgia has become a burden for most patients, it is found that vaccination against herpes zoster among the elderly individuals will provide a cost-effective solution in improving their quality of life.[59] The general population has to be educated regarding the risks and complications of herpes zoster infection so that they can self determine seeking hospital care and getting vaccinated.
Zoster Immune Globulin
Zoster immune globulin is a gamma globulin fraction of plasma obtained from a patient recovering from herpes zoster.[167] It is widely used and is effective in immunocompromised children to provide passive immunization against varicella zoster virus. It has to be administered within 72 hours of exposure to varicella.[167],[168],[169] Normal susceptible children exposed to varicella have also shown effect with zoster immune globulin administration.[170] A study on zoster immune globulin prophylaxis compared with normal serum globulin in immunocompromised patients with disseminated zoster showed the same effect for zoster immune globulin and normal serum globulin in the dissemination rate of herpes zoster and post-herpetic neuralgia.[171] Administration of pooled gamma globulin has shown a reduction in zoster infection.[172],[173] Zoster immune globulin has provided an effective post-exposure prophylaxis in preventing or modifying varicella zoster virus. However, its effect on herpes zoster virus is unclear.
Conclusion
Herpes zoster can affect any age group with a higher incidence in elderly patients and in those with immunocompromised status. The requirement for hospitalization and complications associated with herpes zoster increase with advancing age. More studies are required to confirm the gender specificity, seasonal variations, and regional distribution of herpes zoster. Treatment with antivirals within 72 hours of onset of rash has shown a reduction in herpes zoster and its complications. Famciclovir is found to be superior to valacyclovir. Oral acyclovir and valacyclovir is preferred over famciclovir for patients with acute kidney injury. Pregnant women, children, and immunocompromised patients respond well to acyclovir. Pregabalin and gabapentin along with oxycodone, vitamin C infusion, and MeB12 infusion have shown significant effect in the treatment of post-herpetic neuralgia. Spinal cord stimulation is effective in reducing and preventing post-herpetic neuralgia but at an increased cost. Acute zoster pain can be reduced with epidural anesthetics and steroids. More studies are required for the efficacy of cryotherapy of the intercostal nerves and thalamic stimulation in preventing post-herpetic neuralgia. Herpes zoster vaccination for individuals aged ≥60 years reduces the incidence, burden of illness, and morbidity associated with herpes zoster and post-herpetic neuralgia. Post-exposure prophylaxis of zoster immune globulin for herpes zoster is unclear. Despite several therapeutic modalities for herpes zoster and its complications, the treatment remains a challenge.
Acknowledgement
Author would like to thank Dr. Rahmathullah M.N and Dr. Saramba M.I for their support in reviewing the article for submission.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Gnann JW Jr., Whitley RJ. Clinical practice. Herpes zoster. N Engl J Med 2002;347:340-6.
[Google Scholar]
|
| 2. |
Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015;372:2087-96.
[Google Scholar]
|
| 3. |
Gershon AA, Gershon MD, Breuer J, Levin MJ, Oaklander AL, Griffiths PD, et al. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J Clin Virol 2010;48 Suppl 1:S2-7.
[Google Scholar]
|
| 4. |
Ono F, Yasumoto S, Furumura M, Hamada T, Ishii N, Gyotoku T, et al. Comparison between famciclovir and valacyclovir for acute pain in adult japanese immunocompetent patients with herpes zoster. J Dermatol 2012;39:902-8.
[Google Scholar]
|
| 5. |
Ansaldi F, Trucchi C, Alicino C, Paganino C, Orsi A, Icardi G, et al. Real-world effectiveness and safety of a live-attenuated herpes zoster vaccine: A Comprehensive review. Adv Ther 2016;33:1094-104.
[Google Scholar]
|
| 6. |
Pentikis HS, Matson M, Atiee G, Boehlecke B, Hutchins JT, Patti JM, et al. Pharmacokinetics and safety of FV-100, a novel oral anti-herpes zoster nucleoside analogue, administered in single and multiple doses to healthy young adult and elderly adult volunteers. Antimicrob Agents Chemother 2011;55:2847-54.
[Google Scholar]
|
| 7. |
Schmid DS, Jumaan AO. Impact of varicella vaccine on varicella-zoster virus dynamics. Clin Microbiol Rev 2010;23:202-17.
[Google Scholar]
|
| 8. |
Ogunjimi B, Van Damme P, Beutels P. Herpes zoster risk reduction through exposure to chickenpox patients: A Systematic multidisciplinary review. PLoS One 2013;8:e66485.
[Google Scholar]
|
| 9. |
Mali S. Herpes zoster: Etiology, clinical features and treatment options, and case report. Maxillofac Surg 2012;3:91-100.
[Google Scholar]
|
| 10. |
Johnson RW. Herpes zoster and postherpetic neuralgia. Expert Rev Vaccines 2010;9:21-6.
[Google Scholar]
|
| 11. |
Gabutti G, Franco E, Bonanni P, Conversano M, Ferro A, Lazzari M, et al. Reducing the burden of herpes zoster in italy. Hum Vaccin Immunother 2015;11:101-7.
[Google Scholar]
|
| 12. |
Strommen GL, Pucino F, Tight RR, Beck CL. Human infection with herpes zoster: Etiology, pathophysiology, diagnosis, clinical course, and treatment. Pharmacotherapy 1988;8:52-68.
[Google Scholar]
|
| 13. |
Arvin A. Aging, immunity, and the varicella-zoster virus. N Engl J Med 2005;352:2266-7.
[Google Scholar]
|
| 14. |
Brisson M, Gay NJ, Edmunds WJ, Andrews NJ. Exposure to varicella boosts immunity to herpes-zoster: Implications for mass vaccination against chickenpox. Vaccine 2002;20:2500-7.
[Google Scholar]
|
| 15. |
Johnson RW, Alvarez-Pasquin MJ, Bijl M, Franco E, Gaillat J, Clara JG, et al. Herpes zoster epidemiology, management, and disease and economic burden in europe: A multidisciplinary perspective. Ther Adv Vaccines 2015;3:109-20.
[Google Scholar]
|
| 16. |
Wehrhahn MC, Dwyer DE. Herpes zoster: Epidemiology, clinical features, treatment and prevention. Aust Prescr 2012;35:143-7.
[Google Scholar]
|
| 17. |
Diez-Domingo J, Weinke T, Garcia de Lomas J, Meyer CU, Bertrand I, Eymin C, et al. Comparison of intramuscular and subcutaneous administration of a herpes zoster live-attenuated vaccine in adults aged ≥50 years: A randomised non-inferiority clinical trial. Vaccine 2015;33:789-95.
[Google Scholar]
|
| 18. |
Jeon YH. Herpes zoster and postherpetic neuralgia: Practical consideration for prevention and treatment. Korean J Pain 2015;28:177-84.
[Google Scholar]
|
| 19. |
Fields HL, Rowbotham M, Baron R. Postherpetic neuralgia: Irritable nociceptors and deafferentation. Neurobiol Dis 1998;5:209-27.
[Google Scholar]
|
| 20. |
Baron R. Mechanisms of postherpetic neuralgia – We are hot on the scent. Pain 2008;140:395-6.
[Google Scholar]
|
| 21. |
Johnson RW, Wasner G, Saddier P, Baron R. Herpes zoster and postherpetic neuralgia: Optimizing management in the elderly patient. Drugs Aging 2008;25:991-1006.
[Google Scholar]
|
| 22. |
Bonanni P, Breuer J, Gershon A, Gershon M, Hryniewicz W, Papaevangelou V, et al. Varicella vaccination in Europe – Taking the practical approach. BMC Med 2009;7:26.
[Google Scholar]
|
| 23. |
Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: Towards a global perspective. BMJ Open 2014;4:e004833.
[Google Scholar]
|
| 24. |
Gialloreti LE, Merito M, Pezzotti P, Naldi L, Gatti A, Beillat M, et al. Epidemiology and economic burden of herpes zoster and post-herpetic neuralgia in italy: A retrospective, population-based study. BMC Infect Dis 2010;10:230.
[Google Scholar]
|
| 25. |
di Luzio Paparatti U, Arpinelli F, Visonà G. Herpes zoster and its complications in italy: An observational survey. J Infect 1999;38:116-20.
[Google Scholar]
|
| 26. |
Di Legami V, Gianino MM, Ciofi degli Atti M, Massari M, Migliardi A, Tomba GS, et al. Epidemiology and costs of herpes zoster: Background data to estimate the impact of vaccination. Vaccine 2007;25:7598-604.
[Google Scholar]
|
| 27. |
Insinga RP, Itzler RF, Pellissier JM, Saddier P, Nikas AA. The incidence of herpes zoster in a united states administrative database. J Gen Intern Med 2005;20:748-53.
[Google Scholar]
|
| 28. |
Johnson BH, Palmer L, Gatwood J, Lenhart G, Kawai K, Acosta CJ, et al. Annual incidence rates of herpes zoster among an immunocompetent population in the united states. BMC Infect Dis 2015;15:502.
[Google Scholar]
|
| 29. |
Chapman RS, Cross KW, Fleming DM. The incidence of shingles and its implications for vaccination policy. Vaccine 2003;21:2541-7.
[Google Scholar]
|
| 30. |
Gauthier A, Breuer J, Carrington D, Martin M, Rémy V. Epidemiology and cost of herpes zoster and post-herpetic neuralgia in the united kingdom. Epidemiol Infect 2009;137:38-47.
[Google Scholar]
|
| 31. |
de Melker H, Berbers G, Hahné S, Rümke H, van den Hof S, de Wit A, et al. The epidemiology of varicella and herpes zoster in the Netherlands: Implications for varicella zoster virus vaccination. Vaccine 2006;24:3946-52.
[Google Scholar]
|
| 32. |
Pierik JG, Gumbs PD, Fortanier SA, Van Steenwijk PC, Postma MJ. Epidemiological characteristics and societal burden of varicella zoster virus in the netherlands. BMC Infect Dis 2012;12:110.
[Google Scholar]
|
| 33. |
Liesegang TJ. Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology 2008;115:S3-12.
[Google Scholar]
|
| 34. |
Kim YJ, Lee CN, Lim CY, Jeon WS, Park YM. Population-based study of the epidemiology of herpes zoster in korea. J Korean Med Sci 2014;29:1706-10.
[Google Scholar]
|
| 35. |
Insinga RP, Itzler RF, Pellissier JM. Acute/subacute herpes zoster: Healthcare resource utilisation and costs in a group of US health plans. Pharmacoeconomics 2007;25:155-69.
[Google Scholar]
|
| 36. |
Stein AN, Britt H, Harrison C, Conway EL, Cunningham A, Macintyre CR, et al. Herpes zoster burden of illness and health care resource utilisation in the australian population aged 50 years and older. Vaccine 2009;27:520-9.
[Google Scholar]
|
| 37. |
Ghaznawi N, Virdi A, Dayan A, Hammersmith KM, Rapuano CJ, Laibson PR, et al. Herpes zoster ophthalmicus: Comparison of disease in patients 60 years and older versus younger than 60 years. Ophthalmology 2011;118:2242-50.
[Google Scholar]
|
| 38. |
Li Y, An Z, Yin D, Liu Y, Huang Z, Xu J, et al. Disease Burden due to herpes zoster among population aged ≥50 years old in China: A community based retrospective survey. PLoS One 2016;11:e0152660.
[Google Scholar]
|
| 39. |
Fleming DM, Cross KW, Cobb WA, Chapman RS. Gender difference in the incidence of shingles. Epidemiol Infect 2004;132:1-5.
[Google Scholar]
|
| 40. |
Ragozzino MW, Melton LJ 3rd, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Medicine (Baltimore) 1982;61:310-6.
[Google Scholar]
|
| 41. |
Goh CL, Khoo L. A retrospective study of the clinical presentation and outcome of herpes zoster in a tertiary dermatology outpatient referral clinic. Int J Dermatol 1997;36:667-72.
[Google Scholar]
|
| 42. |
Chidiac C, Bruxelle J, Daures JP, Hoang-Xuan T, Morel P, Leplège A, et al. Characteristics of patients with herpes zoster on presentation to practitioners in france. Clin Infect Dis 2001;33:62-9.
[Google Scholar]
|
| 43. |
Dubey AK, Jaisankar TJ, Thappa DM. Clinical and morphological characteristics of herpes zoster in South India. Indian J Dermatol 2005;50:203-7.
[Google Scholar]
|
| 44. |
Kayastha BMM, Shrestha P, Shrestha R, Lama L. Changing profile of herpes zoster in Nepal: A hospital-based study. NJDVL 2009;8:1-4.
[Google Scholar]
|
| 45. |
Suhail M, Ejaz A, Abbas M, Naz S, Suhail T. Herpes zoster: Seasonal variations and morphological patterns in Pakistan. JPAD 2011;21:22-6.
[Google Scholar]
|
| 46. |
Hong MJ, Kim YD, Cheong YK, Park SJ, Choi SW, Hong HJ, et al. Epidemiology of postherpetic neuralgia in Korea: An electronic population health insurance system based study. Medicine (Baltimore) 2016;95:e3304.
[Google Scholar]
|
| 47. |
Salleras L, Domínguez A, Vidal J, Plans P, Salleras M, Taberner JL, et al. Seroepidemiology of varicella-zoster virus infection in Catalonia (Spain). Rationale for universal vaccination programmes. Vaccine 2000;19:183-8.
[Google Scholar]
|
| 48. |
Heininger U, Braun-Fahrländer C, Desgrandchamps D, Glaus J, Grize L, Wutzler P, et al. Seroprevalence of varicella-zoster virus immunoglobulin G antibodies in swiss adolescents and risk factor analysis for seronegativity. Pediatr Infect Dis J 2001;20:775-8.
[Google Scholar]
|
| 49. |
Chen SY, Suaya JA, Li Q, Galindo CM, Misurski D, Burstin S, et al. Incidence of herpes zoster in patients with altered immune function. Infection 2014;42:325-34.
[Google Scholar]
|
| 50. |
Buchbinder SP, Katz MH, Hessol NA, Liu JY, O'Malley PM, Underwood R, et al. Herpes zoster and human immunodeficiency virus infection. J Infect Dis 1992;166:1153-6.
[Google Scholar]
|
| 51. |
Yenikomshian MA, Guignard AP, Haguinet F, LaCasce AS, Skarin AT, Trahey A, et al. The epidemiology of herpes zoster and its complications in medicare cancer patients. BMC Infect Dis 2015;15:106.
[Google Scholar]
|
| 52. |
Megna M, Napolitano M, Ayala F, Balato N. The risk of herpes zoster in patients with psoriasis: A retrospective records-based observational study. Indian J Dermatol Venereol Leprol 2016;82:744.
[Google Scholar]
|
| 53. |
Hata A, Kuniyoshi M, Ohkusa Y. Risk of herpes zoster in patients with underlying diseases: A retrospective hospital-based cohort study. Infection 2011;39:537-44.
[Google Scholar]
|
| 54. |
Heymann AD, Chodick G, Karpati T, Kamer L, Kremer E, Green MS, et al. Diabetes as a risk factor for herpes zoster infection: Results of a population-based study in israel. Infection 2008;36:226-30.
[Google Scholar]
|
| 55. |
Forbes HJ, Thomas SL, Langan SM. The epidemiology and prevention of herpes zoster. Curr Dermatol Rep 2012;1:39-47.
[Google Scholar]
|
| 56. |
Yang YW, Chen YH, Wang KH, Wang CY, Lin HW. Risk of herpes zoster among patients with chronic obstructive pulmonary disease: A population-based study. CMAJ 2011;183:E275-80.
[Google Scholar]
|
| 57. |
Yang YW, Chen YH, Lin HW. Risk of herpes zoster among patients with psychiatric diseases: A population-based study. J Eur Acad Dermatol Venereol 2011;25:447-53.
[Google Scholar]
|
| 58. |
Katz J, Cooper EM, Walther RR, Sweeney EW, Dworkin RH. Acute pain in herpes zoster and its impact on health-related quality of life. Clin Infect Dis 2004;39:342-8.
[Google Scholar]
|
| 59. |
Edmunds WJ, Brisson M, Rose JD. The epidemiology of herpes zoster and potential cost-effectiveness of vaccination in england and wales. Vaccine 2001;19:3076-90.
[Google Scholar]
|
| 60. |
Drolet M, Brisson M, Schmader K, Levin M, Johnson R, Oxman M, et al. Predictors of postherpetic neuralgia among patients with herpes zoster: A prospective study. J Pain 2010;11:1211-21.
[Google Scholar]
|
| 61. |
Abdul Latheef EN, Pavithran K. Herpes zoster: A clinical study in 205 patients. Indian J Dermatol 2011;56:529-32.
[Google Scholar]
|
| 62. |
Park SY, Kim JY, Kim CD, Kim CW, Lee KS. A clinical study on herpes zoster during the last 10 year period (1994-2003). Korean J Dermatol 2004;42:1531-5.
[Google Scholar]
|
| 63. |
O'Connor KM, Paauw DS. Herpes zoster. Med Clin North Am 2013;97:503-22, ix.
[Google Scholar]
|
| 64. |
Shin DH, Kim BR, Shin JE, Kim CH. Clinical manifestations in patients with herpes zoster oticus. Eur Arch Otorhinolaryngol 2016;273:1739-43.
[Google Scholar]
|
| 65. |
Coulson S, Croxson GR, Adams R, Oey V. Prognostic factors in herpes zoster oticus (ramsay hunt syndrome). Otol Neurotol 2011;32:1025-30.
[Google Scholar]
|
| 66. |
Kim CH, Choi H, Shin JE. Characteristics of hearing loss in patients with herpes zoster oticus. Medicine (Baltimore) 2016;95:e5438.
[Google Scholar]
|
| 67. |
Aydoǧdu İ, Ataç E, Saltürk Z, Atar Y, Özdemir E, Uyar Y, et al. Pediatric ramsay hunt syndrome: Analysis of three cases. Case Rep Otolaryngol 2015;2015:971249.
[Google Scholar]
|
| 68. |
Schaftenaar E, Meenken C, Baarsma GS, McIntyre JA, Verjans GM, Peters RP, et al. Early- and late-stage ocular complications of herpes zoster ophthalmicus in rural South Africa. Trop Med Int Health 2016;21:334-9.
[Google Scholar]
|
| 69. |
Cappuzzo KA. Treatment of postherpetic neuralgia: Focus on pregabalin. Clin Interv Aging 2009;4:17-23.
[Google Scholar]
|
| 70. |
Volpi A. Severe complications of herpes zoster. Herpes 2007;14 Suppl 2:35-9.
[Google Scholar]
|
| 71. |
Cox BF, Bürgelová M, Veselý D, Tomíčková D, Holub M. Gangrenous herpes zoster with multidermatomal involvement in a patient after kidney transplantation. Prague Med Rep 2011;112:44-9.
[Google Scholar]
|
| 72. |
Kutlubay Z, Göksügür N, Engin B, Tüzün Y. Complications of herpes zoster. J Turk Acad Dermatol 2011;5:115-21.
[Google Scholar]
|
| 73. |
Sewell GS, Hsu VP, Jones SR. Zoster gangrenosum: Necrotizing fasciitis as a complication of herpes zoster. Am J Med 2000;108:520-1.
[Google Scholar]
|
| 74. |
Shaikh S, Ta CN. Evaluation and management of herpes zoster ophthalmicus. Am Fam Physician 2002;66:1723-30.
[Google Scholar]
|
| 75. |
Kalogeropoulos CD, Bassukas ID, Moschos MM, Tabbara KF. Eye and periocular skin involvement in herpes zoster infection. Med Hypothesis Discov Innov Ophthalmol 2015;4:142-56.
[Google Scholar]
|
| 76. |
Tran KD, Falcone MM, Choi DS, Goldhardt R, Karp CL, Davis JL, et al. Epidemiology of herpes zoster ophthalmicus: Recurrence and chronicity. Ophthalmology 2016;123:1469-75.
[Google Scholar]
|
| 77. |
Ong OL, Churchyard AC, New PW. The importance of early diagnosis of herpes zoster myelitis. Med J Aust 2010;193:546-7.
[Google Scholar]
|
| 78. |
Ruiz Junior FB, Shinosaki JS, Marques Junior W, Ferreira MS. Abdominal wall protrusion following herpes zoster. Rev Soc Bras Med Trop 2007;40:234-5.
[Google Scholar]
|
| 79. |
Chernev I, Dado D. Segmental zoster abdominal paresis (zoster pseudohernia): A review of the literature. PM R 2013;5:786-90.
[Google Scholar]
|
| 80. |
Al Rakban A, Siddiqui M, Awada A, Dean C. Abdominal muscle paralysis in herpes zoster. Neurosciences (Riyadh) 2000;5:66-8.
[Google Scholar]
|
| 81. |
Teo HK, Chawla M, Kaushik M. A rare complication of herpes zoster: Segmental zoster paresis. Case Rep Med 2016.
[Google Scholar]
|
| 82. |
Rothrock JF, Walicke PA, Swenson MR. Neurogenic bladder from occult herpes zoster. Postgrad Med 1986;80:211-3, 216.
[Google Scholar]
|
| 83. |
Jakubovicz D, Solway E, Orth P. Herpes zoster: Unusual cause of acute urinary retention and constipation. Can Fam Physician 2013;59:e146-7.
[Google Scholar]
|
| 84. |
Roxas M. Herpes zoster and postherpetic neuralgia: Diagnosis and therapeutic considerations. Altern Med Rev 2006;11:102-13.
[Google Scholar]
|
| 85. |
Lam NN, Weir MA, Yao Z, Blake PG, Beyea MM, Gomes T, et al. Risk of acute kidney injury from oral acyclovir: A population-based study. Am J Kidney Dis 2013;61:723-9.
[Google Scholar]
|
| 86. |
Rasi A, Heshmatzade Behzadi A, Rabet M, Hassanloo J, Honarbakhsh Y, Dehghan N, et al. The efficacy of time-based short-course acyclovir therapy in treatment of post-herpetic pain. J Infect Dev Ctries 2010;4:754-60.
[Google Scholar]
|
| 87. |
Jalali MH, Ansarin H, Soltani-Arabshahi R. Broad-band ultraviolet B phototherapy in zoster patients may reduce the incidence and severity of postherpetic neuralgia. Photodermatol Photoimmunol Photomed 2006;22:232-7.
[Google Scholar]
|
| 88. |
Harding SP, Porter SM. Oral acyclovir in herpes zoster ophthalmicus. Curr Eye Res 1991;10 Suppl: 177-82.
[Google Scholar]
|
| 89. |
Cobo LM, Foulks GN, Liesegang T, Lass J, Sutphin JE, Wilhelmus K, et al. Oral acyclovir in the treatment of acute herpes zoster ophthalmicus. Ophthalmology 1986;93:763-70.
[Google Scholar]
|
| 90. |
Aylward GW, Claoué CM, Marsh RJ, Yasseem N. Influence of oral acyclovir on ocular complications of herpes zoster ophthalmicus. Eye (Lond) 1994;8(Pt 1):70-4.
[Google Scholar]
|
| 91. |
Neoh C, Harding SP, Saunders D, Wallis S, Tullo AB, Nylander A, et al. Comparison of topical and oral acyclovir in early herpes zoster ophthalmicus. Eye (Lond) 1994;8(Pt 6):688-91.
[Google Scholar]
|
| 92. |
Park KY, Han TY, Kim IS, Yeo IK, Kim BJ, Kim MN, et al. The effects of 830 nm light-emitting diode therapy on acute herpes zoster ophthalmicus: A pilot study. Ann Dermatol 2013;25:163-7.
[Google Scholar]
|
| 93. |
Sy A, McLeod SD, Cohen EJ, Margolis TP, Mannis MJ, Lietman TM, et al. Practice patterns and opinions in the management of recurrent or chronic herpes zoster ophthalmicus. Cornea 2012;31:786-90.
[Google Scholar]
|
| 94. |
Aggarwal S, Cavalcanti BM, Pavan-Langston D. Treatment of pseudodendrites in herpes zoster ophthalmicus with topical ganciclovir 0.15% gel. Cornea 2014;33:109-13.
[Google Scholar]
|
| 95. |
Uri N, Greenberg E, Kitzes-Cohen R, Doweck I. Acyclovir in the treatment of ramsay hunt syndrome. Otolaryngol Head Neck Surg 2003;129:379-81.
[Google Scholar]
|
| 96. |
Worme M, Chada R, Lavallee L. An unexpected case of ramsay hunt syndrome: Case report and literature review. BMC Res Notes 2013;6:337.
[Google Scholar]
|
| 97. |
Monsanto RD, Bittencourt AG, Bobato Neto NJ, Beilke SC, Lorenzetti FT, Salomone R, et al. Treatment and prognosis of facial palsy on ramsay hunt syndrome: Results based on a review of the literature. Int Arch Otorhinolaryngol 2016;20:394-400.
[Google Scholar]
|
| 98. |
Murakami S, Hato N, Horiuchi J, Honda N, Gyo K, Yanagihara N, et al. Treatment of ramsay hunt syndrome with acyclovir-prednisone: Significance of early diagnosis and treatment. Ann Neurol 1997;41:353-7.
[Google Scholar]
|
| 99. |
Shin DW, Kim JM, Ahn SW, Youn YC, Kwon OS. Ramsay hunt syndrome after cervical zoster in an immunocompetent patient. Neurol Sci 2014;35:1635-6.
[Google Scholar]
|
| 100. |
Whitley RJ, Weiss H, Gnann JW Jr., Tyring S, Mertz GJ, Pappas PG, et al. Acyclovir with and without prednisone for the treatment of herpes zoster. A randomized, placebo-controlled trial. The national institute of allergy and infectious diseases collaborative antiviral study group. Ann Intern Med 1996;125:376-83.
[Google Scholar]
|
| 101. |
Wood MJ, Johnson RW, McKendrick MW, Taylor J, Mandal BK, Crooks J, et al. Arandomized trial of acyclovir for 7 days or 21 days with and without prednisolone for treatment of acute herpes zoster. N Engl J Med 1994;330:896-900.
[Google Scholar]
|
| 102. |
Clemmensen OJ, Andersen KE. ACTH versus prednisone and placebo in herpes zoster treatment. Clin Exp Dermatol 1984;9:557-63.
[Google Scholar]
|
| 103. |
Elliott FA. Treatment of herpes zoster with high doses of prednisone. Lancet 1964;2:610-1.
[Google Scholar]
|
| 104. |
Eaglstein WH, Katz R, Brown JA. The effects of early corticosteroid therapy on the skin eruption and pain of herpes zoster. JAMA 1970;211:1681-3.
[Google Scholar]
|
| 105. |
Linnemann CC Jr., Biron KK, Hoppenjans WG, Solinger AM. Emergence of acyclovir-resistant varicella zoster virus in an AIDS patient on prolonged acyclovir therapy. AIDS 1990;4:577-9.
[Google Scholar]
|
| 106. |
Cvjetković D, Jovanović J, Hrnjaković-Cvjetković I, Brkić S, Bogdanović M. [Reactivation of herpes zoster infection by varicella-zoster virus]. Med Pregl 1999;52:125-8.
[Google Scholar]
|
| 107. |
Breton G, Fillet AM, Katlama C, Bricaire F, Caumes E. Acyclovir-resistant herpes zoster in human immunodeficiency virus-infected patients: Results of foscarnet therapy. Clin Infect Dis 1998;27:1525-7.
[Google Scholar]
|
| 108. |
Blot N, Schneider P, Young P, Janvresse C, Dehesdin D, Tron P, et al. Treatment of an acyclovir and foscarnet-resistant herpes simplex virus infection with cidofovir in a child after an unrelated bone marrow transplant. Bone Marrow Transplant 2000;26:903-5.
[Google Scholar]
|
| 109. |
Alrabiah FA, Sacks SL. New antiherpesvirus agents. Their targets and therapeutic potential. Drugs 1996;52:17-32.
[Google Scholar]
|
| 110. |
Rossi S, Whitfeld M, Berger TG. The treatment of acyclovir-resistant herpes zoster with trifluorothymidine and interferon alfa. Arch Dermatol 1995;131:24-6.
[Google Scholar]
|
| 111. |
Pasternak B, Hviid A. Use of acyclovir, valacyclovir, and famciclovir in the first trimester of pregnancy and the risk of birth defects. JAMA 2010;304:859-66.
[Google Scholar]
|
| 112. |
Ratanajamit C, Vinther Skriver M, Jepsen P, Chongsuvivatwong V, Olsen J, Sørensen HT, et al. Adverse pregnancy outcome in women exposed to acyclovir during pregnancy: A population-based observational study. Scand J Infect Dis 2003;35:255-9.
[Google Scholar]
|
| 113. |
Sadati MS, Ahrari I. Severe herpes zoster neuralgia in a pregnant woman treated with acetaminophen. Acta Med Iran 2014;52:238-9.
[Google Scholar]
|
| 114. |
Daïen CI, Cohen JD, Jorgensen C. Zoster cruralgia in a pregnant woman. Joint Bone Spine 2009;76:724-5.
[Google Scholar]
|
| 115. |
Marin M, Güris D, Chaves SS, Schmid S, Seward JF, Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). et al. Prevention of varicella: Recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep 2007;56:1-40.
[Google Scholar]
|
| 116. |
Rothe MJ, Feder HM Jr., Grant-Kels JM. Oral acyclovir therapy for varicella and zoster infections in pediatric and pregnant patients: A brief review. Pediatr Dermatol 1991;8:236-42, 246-7.
[Google Scholar]
|
| 117. |
Kakourou T, Theodoridou M, Mostrou G, Syriopoulou V, Papadogeorgaki H, Constantopoulos A, et al. Herpes zoster in children. J Am Acad Dermatol 1998;39:207-10.
[Google Scholar]
|
| 118. |
Kawasaki H, Takayama J, Ohira M. Herpes zoster infection after bone marrow transplantation in children. J Pediatr 1996;128:353-6.
[Google Scholar]
|
| 119. |
Brar BK, Pall A, Gupta RR. Herpes zoster neonatorum. J Dermatol 2003;30:346-7.
[Google Scholar]
|
| 120. |
Kurlan JG, Connelly BL, Lucky AW. Herpes zoster in the first year of life following postnatal exposure to varicella-zoster virus: Four case reports and a review of infantile herpes zoster. Arch Dermatol 2004;140:1268-72.
[Google Scholar]
|
| 121. |
Harpaz R, Ortega-Sanchez IR, Seward JF, Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: Recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep 2008;57:1-30.
[Google Scholar]
|
| 122. |
Balfour HH Jr., Bean B, Laskin OL, Ambinder RF, Meyers JD, Wade JC, et al. Acyclovir halts progression of herpes zoster in immunocompromised patients. N Engl J Med 1983;308:1448-53.
[Google Scholar]
|
| 123. |
Santos KB, Souza RS, Atalla A, Hallack-Neto AE. Herpes zoster after autologous hematopoietic stem cell transplantation. Rev Bras Hematol Hemoter 2016;38:298-301.
[Google Scholar]
|
| 124. |
Breton G, Bouldouyre MA, Gervais A, Duval X, Longuet P, Leport C, et al. Failure of valacyclovir for herpes zoster in a moderately immunocompromised HIV-infected patient. AIDS Patient Care STDS 2004;18:255-7.
[Google Scholar]
|
| 125. |
Kim SJ, Kim K, Do YR, Bae SH, Yang DH, Lee JJ, et al. Low-dose acyclovir is effective for prevention of herpes zoster in myeloma patients treated with bortezomib: A report from the korean multiple myeloma working party (KMMWP) retrospective study. Jpn J Clin Oncol 2011;41:353-7.
[Google Scholar]
|
| 126. |
Barnabas RV, Baeten JM, Lingappa JR, Thomas KK, Hughes JP, Mugo NR, et al. Acyclovir prophylaxis reduces the incidence of herpes zoster among HIV-infected individuals: Results of a randomized clinical trial. J Infect Dis 2016;213:551-5.
[Google Scholar]
|
| 127. |
Levin MJ, Zaia JA, Hershey BJ, Davis LG, Robinson GV, Segreti AC, et al. Topical acyclovir treatment of herpes zoster in immunocompromised patients. J Am Acad Dermatol 1985;13:590-6.
[Google Scholar]
|
| 128. |
Wutzler P, De Clercq E, Wutke K, Färber I. Oral brivudin vs. Intravenous acyclovir in the treatment of herpes zoster in immunocompromised patients: A randomized double-blind trial. J Med Virol 1995;46:252-7.
[Google Scholar]
|
| 129. |
Arora A, Mendoza N, Brantley J, Yates B, Dix L, Tyring S, et al. Double-blind study comparing 2 dosages of valacyclovir hydrochloride for the treatment of uncomplicated herpes zoster in immunocompromised patients 18 years of age and older. J Infect Dis 2008;197:1289-95.
[Google Scholar]
|
| 130. |
Tyring S, Belanger R, Bezwoda W, Ljungman P, Boon R, Saltzman RL, et al. Arandomized, double-blind trial of famciclovir versus acyclovir for the treatment of localized dermatomal herpes zoster in immunocompromised patients. Cancer Invest 2001;19:13-22.
[Google Scholar]
|
| 131. |
Vickrey E, Allen S, Mehta J, Singhal S. Acyclovir to prevent reactivation of varicella zoster virus (herpes zoster) in multiple myeloma patients receiving bortezomib therapy. Cancer 2009;115:229-32.
[Google Scholar]
|
| 132. |
Sayanlar J, Guleyupoglu N, Portenoy R, Ashina S. Trigeminal postherpetic neuralgia responsive to treatment with capsaicin 8 % topical patch: A case report. J Headache Pain 2012;13:587-9.
[Google Scholar]
|
| 133. |
Binder A, Bruxelle J, Rogers P, Hans G, Bösl I, Baron R, et al. Topical 5% lidocaine (lignocaine) medicated plaster treatment for post-herpetic neuralgia: Results of a double-blind, placebo-controlled, multinational efficacy and safety trial. Clin Drug Investig 2009;29:393-408.
[Google Scholar]
|
| 134. |
Davies PS, Galer BS. Review of lidocaine patch 5% studies in the treatment of postherpetic neuralgia. Drugs 2004;64:937-47.
[Google Scholar]
|
| 135. |
Kukkar A, Bali A, Singh N, Jaggi AS. Implications and mechanism of action of gabapentin in neuropathic pain. Arch Pharm Res 2013;36:237-51.
[Google Scholar]
|
| 136. |
Wiffen PJ, Derry S, Moore RA, McQuay HJ. Carbamazepine for acute and chronic pain in adults. Cochrane Database Syst Rev 2011: CD005451.
[Google Scholar]
|
| 137. |
Beydoun A. Postherpetic neuralgia: Role of gabapentin and other treatment modalities. Epilepsia 1999;40 Suppl 6:S51-6.
[Google Scholar]
|
| 138. |
Rauck RL, Irving GA, Wallace MS, Vanhove GF, Sweeney M. Once-daily gastroretentive gabapentin for postherpetic neuralgia: Integrated efficacy, time to onset of pain relief and safety analyses of data from two phase 3, multicenter, randomized, double-blind, placebo-controlled studies. J Pain Symptom Manage 2013;46:219-28.
[Google Scholar]
|
| 139. |
Wang BC, Liu D, Furnback WE, Bifa F, Dong P, Xie L, et al. The cost-effectiveness of pregabalin versus gabapentin for peripheral neuropathic pain (pNeP) and postherpetic neuralgia (PHN) in china. Pain Ther 2016;5:81-91.
[Google Scholar]
|
| 140. |
Pérez C, Navarro A, Saldaña MT, Masramón X, Rejas J. Pregabalin and gabapentin in matched patients with peripheral neuropathic pain in routine medical practice in a primary care setting: Findings from a cost-consequences analysis in a nested case-control study. Clin Ther 2010;32:1357-70.
[Google Scholar]
|
| 141. |
Baron R, Binder A, Wasner G. Neuropathic pain: Diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 2010;9:807-19.
[Google Scholar]
|
| 142. |
Liang L, Li X, Zhang G, Sun Y, Yu H, Jiao J, et al. Pregabalin in the treatment of herpetic neuralgia: Results of a multicenter chinese study. Pain Med 2015;16:160-7.
[Google Scholar]
|
| 143. |
Achar A, Chakraborty PP, Bisai S, Biswas A, Guharay T. Comparative study of clinical efficacy of amitriptyline and pregabalin in postherpetic neuralgia. Acta Dermatovenerol Croat 2012;20:89-94.
[Google Scholar]
|
| 144. |
Xu G, Lv ZW, Feng Y, Tang WZ, Xu GX. A single-center randomized controlled trial of local methylcobalamin injection for subacute herpetic neuralgia. Pain Med 2013;14:884-94.
[Google Scholar]
|
| 145. |
Schencking M, Vollbracht C, Weiss G, Lebert J, Biller A, Goyvaerts B, et al. Intravenous vitamin C in the treatment of shingles: Results of a multicenter prospective cohort study. Med Sci Monit 2012;18:CR215-24.
[Google Scholar]
|
| 146. |
Chen N, Yang M, He L, Zhang D, Zhou M, Zhu C, et al. Corticosteroids for preventing postherpetic neuralgia. Cochrane Database Syst Rev 2010: CD005582.
[Google Scholar]
|
| 147. |
Dureja GP, Usmani H, Khan M, Tahseen M, Jamal A. Efficacy of intrathecal midazolam with or without epidural methylprednisolone for management of post-herpetic neuralgia involving lumbosacral dermatomes. Pain Physician 2010;13:213-21.
[Google Scholar]
|
| 148. |
van Wijck AJ, Opstelten W, Moons KG, van Essen GA, Stolker RJ, Kalkman CJ, et al. The PINE study of epidural steroids and local anaesthetics to prevent postherpetic neuralgia: A randomised controlled trial. Lancet 2006;367:219-24.
[Google Scholar]
|
| 149. |
Pasqualucci A, Pasqualucci V, Galla F, De Angelis V, Marzocchi V, Colussi R, et al. Prevention of post-herpetic neuralgia: Acyclovir and prednisolone versus epidural local anesthetic and methylprednisolone. Acta Anaesthesiol Scand 2000;44:910-8.
[Google Scholar]
|
| 150. |
Green CR, de Rosayro AM, Tait AR. The role of cryoanalgesia for chronic thoracic pain: Results of a long-term follow up. J Natl Med Assoc 2002;94:716-20.
[Google Scholar]
|
| 151. |
Moorjani N, Zhao F, Tian Y, Liang C, Kaluba J, Maiwand MO, et al. Effects of cryoanalgesia on post-thoracotomy pain and on the structure of intercostal nerves: A human prospective randomized trial and a histological study. Eur J Cardiothorac Surg 2001;20:502-7.
[Google Scholar]
|
| 152. |
Detterbeck FC. Efficacy of methods of intercostal nerve blockade for pain relief after thoracotomy. Ann Thorac Surg 2005;80:1550-9.
[Google Scholar]
|
| 153. |
Jones MJ, Murrin KR. Intercostal block with cryotherapy. Ann R Coll Surg Engl 1987;69:261-2.
[Google Scholar]
|
| 154. |
Calandria L. Cryoanalgesia for post-herpetic neuralgia: A new treatment. Int J Dermatol 2011;50:746-50.
[Google Scholar]
|
| 155. |
Harke H, Gretenkort P, Ladleif HU, Koester P, Rahman S. Spinal cord stimulation in postherpetic neuralgia and in acute herpes zoster pain. Anesth Analg 2002;94:694-700.
[Google Scholar]
|
| 156. |
Liu MX, Zhong J, Zhu J, Xia L, Dou NN. Treatment of postherpetic neuralgia using DREZotomy guided by spinal cord stimulation. Stereotact Funct Neurosurg 2015;93:178-81.
[Google Scholar]
|
| 157. |
Baek IY, Park JY, Kim HJ, Yoon JU, Byoen GJ, Kim KH, et al. Spinal cord stimulation in the treatment of postherpetic neuralgia in patients with chronic kidney disease: A case series and review of the literature. Korean J Pain 2011;24:154-7.
[Google Scholar]
|
| 158. |
Meglio M, Cioni B, Prezioso A, Talamonti G. Spinal cord stimulation (SCS) in the treatment of postherpetic pain. Acta Neurochir Suppl (Wien) 1989;46:65-6.
[Google Scholar]
|
| 159. |
Iseki M, Morita Y, Nakamura Y, Ifuku M, Komatsu S. Efficacy of limited-duration spinal cord stimulation for subacute postherpetic neuralgia. Ann Acad Med Singapore 2009;38:1004-6.
[Google Scholar]
|
| 160. |
Moriyama K. Effect of temporary spinal cord stimulation on postherpetic neuralgia in the thoracic nerve area. Neuromodulation 2009;12:39-43.
[Google Scholar]
|
| 161. |
Yanamoto F, Murakawa K. The effects of temporary spinal cord stimulation (or spinal nerve root stimulation) on the management of early postherpetic neuralgia from one to six months of its onset. Neuromodulation 2012;15:151-4.
[Google Scholar]
|
| 162. |
Green AL, Nandi D, Armstrong G, Carter H, Aziz T. Post-herpetic trigeminal neuralgia treated with deep brain stimulation. J Clin Neurosci 2003;10:512-4.
[Google Scholar]
|
| 163. |
Kolšek M. TENS – An alternative to antiviral drugs for acute herpes zoster treatment and postherpetic neuralgia prevention. Swiss Med Wkly 2012;141:w13229.
[Google Scholar]
|
| 164. |
Xu G, Xú G, Feng Y, Tang WZ, Lv ZW. Transcutaneous electrical nerve stimulation in combination with cobalamin injection for postherpetic neuralgia: A single-center randomized controlled trial. Am J Phys Med Rehabil 2014;93:287-98.
[Google Scholar]
|
| 165. |
Boivin G, Jovey R, Elliott CT, Patrick DM. Management and prevention of herpes zoster: A Canadian perspective. Can J Infect Dis Med Microbiol 2010;21:45-52.
[Google Scholar]
|
| 166. |
Oxman MN, Levin MJ, Shingles Prevention Study Group. Vaccination against herpes zoster and postherpetic neuralgia. J Infect Dis 2008;197 Suppl 2:S228-36.
[Google Scholar]
|
| 167. |
Judelsohn RG, Meyers JD, Ellis RJ, Thomas EK. Efficacy of zoster immune globulin. Pediatrics 1974;53:476-80.
[Google Scholar]
|
| 168. |
Gershon AA, Steinberg S, Brunell PA. Zoster immune globulin. A further assessment. N Engl J Med 1974;290:243-5.
[Google Scholar]
|
| 169. |
Brunell PA, Gershon AA, Hughes WT, Riley HD Jr., Smith J. Prevention of varicella in high risk children: A collaborative study. Pediatrics 1972;50:718-22.
[Google Scholar]
|
| 170. |
Brunell PA, Ross A, Miller LH, Kuo B. Prevention of varicella by zoster immune globulin. N Engl J Med 1969;280:1191-4.
[Google Scholar]
|
| 171. |
Stevens DA, Merigan TC. Zoster immune globulin prophylaxis of disseminated zoster in compromised hosts. A randomized trial. Arch Intern Med 1980;140:52-4.
[Google Scholar]
|
| 172. |
Weintraub II. Treatment of herpes zoster with gamma globulin. J Am Med Assoc 1955;157:1611.
[Google Scholar]
|
| 173. |
Rodarte JG, Williams BH. Treatment of herpes zoster and chicken pox with immune globulin. AMA Arch Derm 1956;73:553-5.
[Google Scholar]
|
Fulltext Views
46,402
PDF downloads
9,828





