Translate this page into:
A morphometric and immunohistochemical study of melanocytes in periorbital hyperpigmentation
2 Department of Pathology, Army College of Medical Sciences, New Delhi, India
3 Department of Dermatology, Command Hospital (Eastern Command), Kolkata, West Bengal, India
4 Department of Dermatology, INHS Asvini, Mumbai, Maharashtra, India
Correspondence Address:
Dibyajyoti Boruah
Department of Pathology, Armed Forces Medical College, Pune - 411 040, Maharashtra
India
| How to cite this article: Boruah D, Manu V, Malik A, Chatterjee M, Vasudevan B, Srinivas V. A morphometric and immunohistochemical study of melanocytes in periorbital hyperpigmentation. Indian J Dermatol Venereol Leprol 2015;81:588-593 |
Abstract
Background: An increase in number of melanocytes in the basal cell layer of the epidermis is an important feature in many disorders of hyperpigmentation. In this study, we attempted an objective evaluation of the linear density of melanocytes and keratinocytes, along with other epidermal characteristics, in periorbital hyperpigmentation using immunohistochemistry and morphometric techniques. Methods: Melanocytes and epidermal parameters were assessed by digital morphometry in 30 newly diagnosed cases of periorbital hyperpigmentation and 14 controls from the post-auricular region. Melanocytes were labelled with the immunohistochemical stains, Melan-A and tyrosinase. We studied the linear keratinocyte density, mean linear melanocyte density, ratio of melanocytes to keratinocytes, the ratio between inner and outer epidermal length, maximum epidermal thickness and minimum epidermal thickness. Results: Melan-A expression of melanocytes showed strong positive correlation (r = 0.883) with the tyrosinase expression. Mean linear melanocyte density was 24/mm (range: 13–30/mm) in cases and 17/mm (13–21/mm) in controls and this difference was statistically significant (P < 0.001). The mean ratio of melanocyte to keratinocyte was 0.22 (0.12–0.29) in cases and 0.16 (0.12–0.21) in controls; again, this difference was statistically significant (P < 0.001). There was a mild negative correlation with linear keratinocyte density (r = −0.302) and the ratio between inner and outer epidermal length (r = −0.456). However, there were no differences in epidermal thicknesses. Limitations: There were fewer control biopsies than optimal, and they were not taken from the uninvolved periorbital region. Conclusion: Mean linear melanocyte density and the ratio of melanocytes to keratinocytes is increased in cases with periorbital hyperpigmentation. It is, therefore, likely that increased melanocyte density may be the key factor in the pathogenesis of periorbital hyperpigmentation.Introduction
Skin color is highly variable and different skin colors usually result from the density of epidermal melanocytes and the size and number of melanosomes synthesized in the skin melanocytes.[1] Facial pigmentation is a significant cosmetic problem, especially in women.[2] Exposure to sunlight, genetic factors, the use of cosmetics, chronic inflammation, certain drugs and stress have all been implicated in the pathogenesis of most cases of facial hyperpigmentation.[3] Disorders of pigmentation can also result from abnormalities of melanocyte migration, impairment of melanosome transfer to the surrounding keratinocytes, an alteration in melanin synthesis and a defective degradation or removal of melanin.[4]
Periorbital hyperpigmentation is characterized by dark circles around the eyes which are common, often familial, and frequently found in individuals of darker skin colour.[5],[6] Increase of melanocytes in the basal cell layer of the epidermis is one of the important findings in these cases. In this study, we attempted an objective evaluation of the linear density of melanocytes and keratinocytes along with other epidermal characteristics in the periorbital region using the morphometric technique on immunohistochemical stained sections.
Methods
After obtaining institutional ethical clearance, a total of 30 patients with newly diagnosed hyperpigmentation in the periorbital region, presenting to the dermatology department over a duration of 3 years, were included in the study. Incisional skin biopsies (about 2 mm depth) were taken from the area showing maximum pigmentation. Fourteen control samples were also taken from those patients willing to provide one, Control biopsies were taken from a site in the postauricular region without any skin problem, not covered by hair and exposed to an almost equal amount of sunlight. Informed consent was obtained from all patients. Patients receiving any mode of therapy were excluded. Immunohistochemical staining with Melan-A and tyrosinase antibodies was done to label melanocytes in the skin biopsy. We studied linear keratinocyte density, linear melanocyte density with Melan-A expression, linear melanocyte density with tyrosine expression, mean linear melanocyte density, ratio of melanocyte to keratinocyte, ratio between inner and outer epidermal length, maximum epidermal thickness and minimum epidermal thickness.
Histopathological evaluation and immunohistochemistry for melan-A and tyrosinase
Two pathologists independently reviewed routine hematoxylin and eosin stained slides of all cases. Representative blocks of formalin-fixed paraffin-embedded tissue were selected, 4-micrometer thick sections cut and immunohistochemical staining performed by labeled streptavidin-biotin technique using mouse anti-human Melan-A and tyrosinase monoclonal antibodies. Melanocytes were observed by scanning sections at low power (×100) magnification and the area with the greatest number of Melan-A and tyrosinase immunostained melanocytes ("hot spot") was selected. Melanocyte and keratinocyte counting was done under high power magnification (×400) in five fields within the hot spot (area of each field = 0.0577 mm 2) [Figure - 1]a. A section of a sample stained with hematoxylin and eosin has been shown in [Figure - 1]b. [Figure - 1]c is a tyrosinase-immunostained section of a case with periorbital hyperpigmentation; [Figure - 1]d and e are the Melan-A immunostained sections of a case and control, respectively.
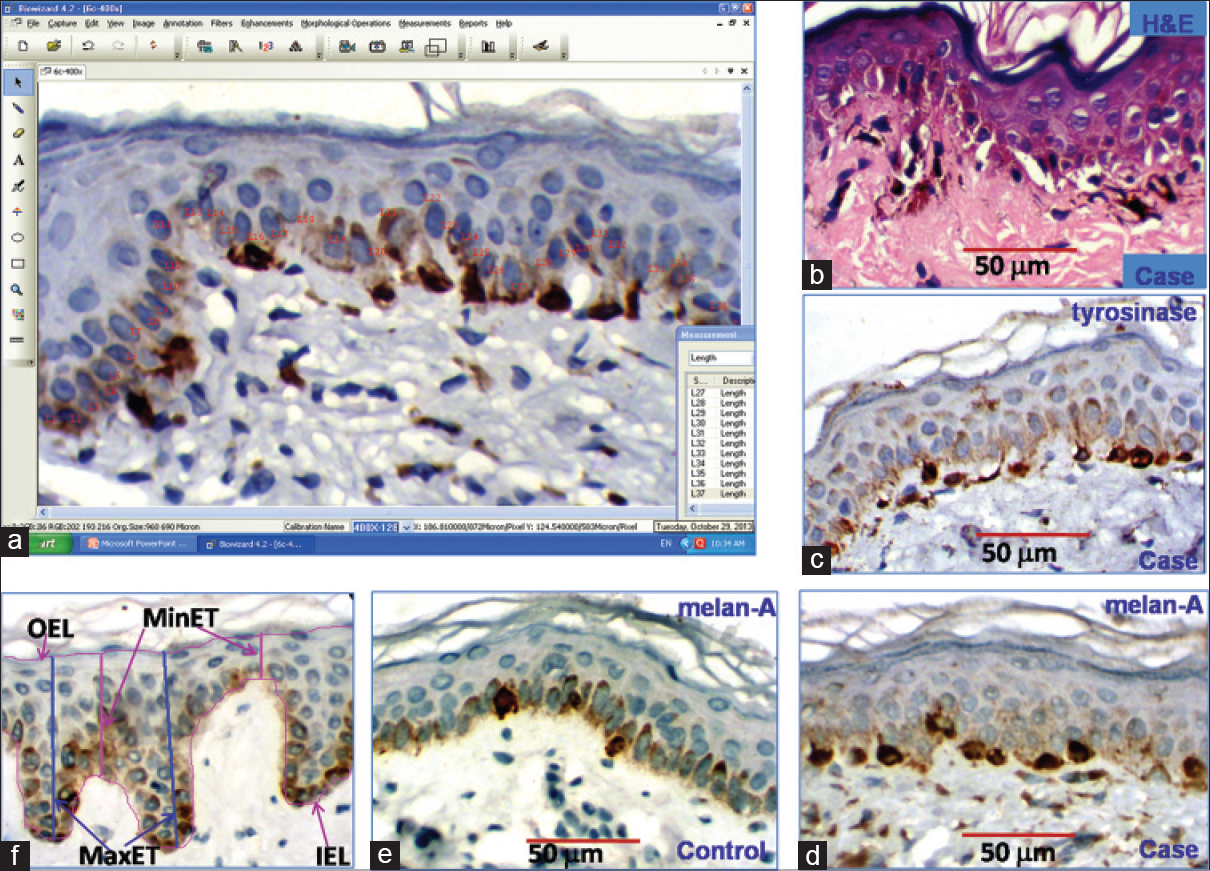 |
| Figure 1: (a) Image morphometric counting of keratinocytes and melanocytes in skin tissue, (b) (H and E) stained section of a sample (×400), (c) tyrosinase expression in a case of periorbital hyperpigmentation (×400), (d and e) Melan-A expression in a case of periorbital hyperpigmentation and a control, respectively (×400) (f) measurement scheme of epidermis parameters: inner epidermal length, outer epidermal length, maximum epidermal thickness and minimum epidermal thickness |
Morphometric analysis
Morphometric analysis was performed on Melan-A and tyrosinase immunostained sections using a computerized digital photomicrography system (Dewinter Optical Inc., with Digi Eye 330 digital photomicrography camera and Biowizard 4.2 Image analysis software (Dewinter Optical, Inc., Delhi, India)) [Figure - 1]a. The measuring scale of image analysis software was properly calibrated with the standard scale, as per the instruction given in the software manual. Images from five high power fields (×400) in the hot spot area were recorded for each sample. Melanocytes and keratinocytes in these fields were counted and the epidermal parameters (epidermal thicknesses, inner epidermal length and outer epidermal length) were evaluated [Figure - 1]f.
The distance from the crest (ridge) of the stratum basale (papillae) to the interface of stratum spinosum and stratum granulosum was considered as maximum epidermal thickness. Similarly, the distance from the trough (trench) of the stratum basale to the interface of stratum spinosum and stratum granulosum was considered as minimum epidermal thickness. Three measurements of maximum and minimum epidermal thickness were taken for each of the five recorded ×400 fields of each sample and their mean value was calculated. The total length of the stratum basale in a ×400 field was taken as the inner epidermal length and the total length of the interface of stratum spinosum and stratum granulosum was considered as the outer epidermal length. Then, the ratio between inner and outer epidermal length was determined for the three recorded fields of each sample and their mean value was determined. The ratio between inner and outer epidermal length is, therefore, a measure of the tortuosity of the basal layer.[7] All these measurements were carried out using image analysis software.
The parameters were evaluated as follows:
- Linear keratinocyte density = (number of keratinocytes in basale layer)/(inner epidermal length)
- Linear melanocyte density with melan-A expression = (number of melanocytes with melan-A expression)/(inner epidermal length)
- Linear melanocyte density with tyrosine expression = (number of melanocytes with tyrosine expression)/(inner epidermal length)
- Mean linear melanocyte density = ([linear melanocyte density with Melan-A expression) + (linear melanocyte density with tyrosine expression])/2
- The ratio of melanocyte to keratinocyte = (mean linear melanocyte density)/(linear keratinocyte density).
Statistical analysis
All parameters were statistically analyzed for each sample. The mean values of these parameters with standard deviation were calculated. Data was reported as mean, standard deviation and range of these parameters. The t-test was performed to evaluate the statistical significance of the difference in parameters between cases and controls. Correlations among parameters were investigated and Pearson correlation coefficient r and P value was calculated with regression lines.
Results
Of the 30 cases, there were 4 males and 26 females; their mean age at the time of biopsy was 30 years (range: 17–49 years). Of the 14 controls, one was male and 13 female; their mean age at the time of biopsy was 31 years (range: 20–48 years). The mean values for cases and controls of all the studied parameters with standard deviation and range are presented in [Table - 1]. Linear melanocyte density with Melan-A expression, linear melanocyte density with tyrosine expression, mean linear melanocyte density and ratio of melanocytes to keratinocytes showed a significant difference (P < 0.001) between cases and controls, whereas linear keratinocyte density, minimum epidermal thickness, maximum epidermal thickness and ratio between inner and outer epidermal length did not reveal any significant difference.

Linear correlation of linear melanocyte density with tyrosine expression with linear melanocyte density with melan-A expression
[Figure - 2] represents the scatter plot of linear melanocyte density with tyrosine expression versus linear melanocyte density with the melan-A expression for all samples with linear regression. A strong positive correlation was observed between these two parameters (r = 0.883).
 |
| Figure 2: Scatter plot of linear melanocyte density with tyrosine expression versus linear melanocyte density with melan-A expression for all studied samples with linear regression shown by the solid line in the plot |
Linear correlation of ratio of melanocytes to keratinocytes with linear keratinocyte density, ratio between inner and outer epidermal length, minimum epidermal thickness and maximum epidermal thickness
[Figure - 3]a and [Figure - 3]b represent the scatter plots of the ratio of melanocytes to keratinocytes versus linear keratinocyte density and ratio between inner and outer epidermal length for all samples with linear regressions. The ratio of melanocytes to keratinocytes showed mild negative correlation with linear keratinocyte density (r = −0.302) and ratio between inner and outer epidermal length (r = −0.456), but no meaningful correlation with minimum epidermal thickness (r = 0.051) and maximum epidermal thickness (r = −0.103).
 |
| Figure 3: (a and b) Scatter plot of ratio of melanocyte to keratinocyte versus: (a) Linear keratinocyte density and (b) ratio between inner and outer epidermal length for all studied samples with linear regression. The linear regressions are shown by the solid lines in their respective plot |
Linear correlation of linear keratinocyte density with minimum epidermal thickness and maximum epidermal thickness
[Figure - 4]a and [Figure - 4]b represent the scatter plots of linear keratinocyte density versus minimum epidermal thickness and maximum epidermal thickness for all samples with linear regressions. Linear keratinocyte density showed a moderate negative correlation with a minimum epidermal thickness (r = −0.603) and mild negative correlation with a maximum epidermal thickness (r = −0.361).
 |
| Figure 4: (a and b) Scatter plot of linear keratinocyte density versus: (a) Minimum epidermal thickness and (b) maximum epidermal thickness for all studied samples; linear regression of these parameters with linear keratinocyte density are shown by the solid lines in their respective plot |
Discussion
Over the years, there has been an increase in patients with aesthetic/cosmetic concerns, especially involving the face. Of these, one of the most common is periorbital hyperpigmentation or dark circles below the eye. Periorbital hyperpigmentation is defined as bilateral, homogeneous, hyperchromic macules and patches primarily involving the lower eyelids but also sometimes extending toward the upper eyelids, eyebrows, malar regions, temporal regions and lateral nasal root.[5],[6],[8] In a recent Indian study, Sheth et al. examined the prevalence and type of periorbital hyperpigmentation, common causative factors and association with personal habits and other disorders within various age and sex groups.[6] In their study, the prevalence of periorbital hyperpigmentation was estimated to be 30.8%, and it was more common in women than in men (4.2:1), and in a younger age group (16–25 years).
The etiology of periorbital hyperpigmentation is multifactorial. The various factors implicated are underlying systemic/metabolic/hormonal diseases, nutritional deficiencies, allergic reactions, aging, sleep disorders, atopic dermatitis, stress, family history, genetic factors, alcohol consumption, smoking, frequent cosmetic use, frequent eye rubbing and lack of correction for errors of refraction like myopia.[5],[6]
The plausible pathogenic mechanisms of the hyperpigmentation include: (a) increased melanocyte density, (b) increased melanocyte to keratinocyte ratio, (c) increased melanin production by melanocytes, (d) increased or excessive transfer of melanin to adjacent keratinocytes, (e) variable melanophage deposits in dermal cells, (f) thin, translucent lower eyelid skin coupled with superficially located prominent capillaries or telangiectasia and (g) shadowing due to skin laxity, an overhanging tarsal muscle, eye bags/periorbital edema, or a deep tear trough.[6],[8],[9],[10],[11]
Clinically, patients of periorbital hyperpigmentation can be classified as constitutional, postinflammatory, vascular, shadow effect and others.[6],[12] Ranu et al., found that the commonest form of periorbital hyperpigmentation in an Asian population was the vascular type 77 (41.8%), followed by constitutional 71 (38.6%), postinflammatory hyperpigmentation 22 (12%) and shadow effects 21 (11.4%).[12] However, Sheth et. al. observed that the commonest form in an Indian population was constitutional 103 (51.5%), followed by postinflammatory hyperpigmentation 45 (22.5%), vascular 16 (8%), shadow 5 (2.5%) and others 31 (15.5%).[6] Since the number of samples in our study was small and our main goal was to determine melanocyte concentration and epidermal characteristics, we did not categorize the cases.
We compared the findings in biopsies from the involved area with control tissue from the postauricular region of the same patient to eliminate individual variation. The ideal comparison would have been between pigmented and normal eyelid skin. Unfortunately, there are practical and ethical difficulties in doing this in a clinical setting as it would be difficult to obtain consent for a biopsy of uninvolved periorbital skin.
In our study, the majority of patients were women and the average age was 30 years, which is comparable to most other studies. We found the melanocytes per unit length of the basal layer to be significantly higher (P < 0.001) in periorbital hyperpigmentation than in controls.
Melan-A labeling and tyrosinase labeling both labeled the melanocytes equally in cases and controls and there was no difference in the staining pattern or intensity.
There was no significant difference in the epidermal thickness parameters in our study, though some authors have suggested thinness of the epidermis as a contributing factor.[13] However, we found that minimum and maximum epidermal thickness showed a negative correlation with linear keratinocyte density, i.e., epidermal thickness showed a decreasing trend with an increase in keratinocyte density. The ratio of melanocytes to keratinocytes also showed a negative correlation with linear keratinocyte density and the ratio between inner and outer epidermal length, i.e., melanocyte to keratinocyte ratio showed a decreasing trend with an increase in keratinocyte density and tortuosity of the basal layer.
Watanabe et al. studied periorbital biopsies of 12 Japanese patients with dark circles under the eyes and showed that all of them had dermal melanocytosis as revealed on histology.[9] We, however, did not find any significant increase in melanocytes or pigmentation in the dermis, either by hematoxylin and eosin staining or by immunohistochemistry. On the contrary, we feel there is an increase in the melanocytes/pigmentation in the epidermis itself. The increase in dermal melanocytes or pigmentation is probably not a significant causative factor in our population, but this needs to be validated with larger samples.
Limitations
There were fewer control biopsies than cases, and were taken from a site in the post-auricular region. The ideal comparison would have been between pigmented and normal skin of the periorbital region in all cases, or from matched healthy controls.
Conclusion
Periorbital hyperpigmentation is a condition with multifactorial etiology and varied pathogenesis. Objective evaluation of epidermal characteristics and melanocyte concentration in such cases can provide a better understanding of the underlying etiopathogenesis. In our study, we found that there were no significant differences in linear keratinocyte density, ratio between inner and outer epidermal length, maximum and minimum epidermal thickness in cases and controls. However, the density of melanocytes and melanocyte to keratinocyte ratio were significantly higher in cases of periorbital hyperpigmentation than in the controls taken from uninvolved postauricular skin.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Costin GE, Hearing VJ. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J 2007;21:976-94.
[Google Scholar]
|
| 2. |
Rigopoulos D, Gregoriou S, Katsambas A. Hyperpigmentation and melasma. J Cosmet Dermatol 2007;6:195-202.
[Google Scholar]
|
| 3. |
Handel AC, Miot LD, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol 2014;89:771-82.
[Google Scholar]
|
| 4. |
Fistarol SK, Itin PH. Disorders of pigmentation. J Dtsch Dermatol Ges 2010;8:187-201.
[Google Scholar]
|
| 5. |
Roberts WE. Periorbital hyperpigmentation: review of etiology, medical evaluation, and aesthetic treatment. J Drugs Dermatol 2014;13:472-82.
[Google Scholar]
|
| 6. |
Sheth PB, Shah HA, Dave JN. Periorbital hyperpigmentation: a study of its prevalence, common causative factors and its association with personal habits and other disorders. Indian J Dermatol 2014;59:151-7.
[Google Scholar]
|
| 7. |
Boruah D, Moorchung N, Vasudevan B, Malik A, Chatterjee M. Morphometric study of microvessels, epidermal characteristics and inflammation in psoriasis vulgaris with their correlations. Indian J Dermatol Venereol Leprol 2013;79:216-23.
[Google Scholar]
|
| 8. |
Freitag FM, Cestari TF. What causes dark circles under the eyes? J Cosmet Dermatol 2007;6:211-5.
[Google Scholar]
|
| 9. |
Watanabe S, Nakai K, Ohnishi T. Condition known as “dark rings under the eyes” in the Japanese population is a kind of dermal melanocytosis which can be successfully treated by Q-switched ruby laser. Dermatol Surg 2006;32:785-9.
[Google Scholar]
|
| 10. |
Khanna N, Rasool S. Facial melanoses: Indian perspective. Indian J Dermatol Venereol Leprol 2011;77:552-63.
[Google Scholar]
|
| 11. |
Roh MR, Chung KY. Infraorbital dark circles: definition, causes, and treatment options. Dermatol Surg 2009;35:1163-71.
[Google Scholar]
|
| 12. |
Ranu H, Thng S, Goh BK, Burger A, Goh CL. Periorbital hyperpigmentation in Asians: an epidemiologic study and a proposed classification. Dermatol Surg 2011;37:1297-303.
[Google Scholar]
|
| 13. |
Epstein JS. Management of infraorbital dark circles. A significant cosmetic concern. Arch Facial Plast Surg 1999;1:303-7.
[Google Scholar]
|
Fulltext Views
2,360
PDF downloads
670





