Translate this page into:
Dermoscopic evaluation of therapeutic response to an intralesional corticosteroid in the treatment of alopecia areata
Correspondence Address:
Devinder Mohan Thappa
Department of Dermatology and STD, Jawaharlal Institute of Postgraduate Medical Education and Research, Pondicherry 605 006
India
| How to cite this article: Ganjoo S, Thappa DM. Dermoscopic evaluation of therapeutic response to an intralesional corticosteroid in the treatment of alopecia areata. Indian J Dermatol Venereol Leprol 2013;79:408-417 |
Abstract
Background: Intralesional corticosteroids are the treatment of choice for adults with less than 50% of scalp area involvement with alopecia areata. The sensitivity of picking up clinical response to treatment by clinical examination is very variable and has inter individual variation. Aims: To evaluate the efficacy of intralesional triamcinolone acetonide in the treatment of alopecia areata and to use dermoscopy to identify signs of early clinical response and adverse effects. Methods: Seventy patches in 60 patients were injected with steroid at 4 weeks interval and followed up for 24 weeks. Treatment response was evaluated using regrowth scale (RGS). Heine DELTA 20; dermatoscope was used to assess disease activity, response to treatment and side effects. Results: Twenty eight patients responded early and achieved RGS of 4 within 12 weeks and 29 patients responded late and achieved RGS of 4 within 24 weeks of initiating therapy. There were 3 patients who did not achieve RGS of 4 at 24 weeks. Late and incomplete responders showed statistically significant association with family history of alopecia areata (p < 0.0001), presence of recurrent disease (p = 0.0147) and presence of nail changes (p = 0.0007). Dermoscopically, 60 patches demonstrated regrowth of new vellus hair at 4 weeks. Tapering hair disappeared maximally at 4 weeks. At 12 weeks, complete disappearance was seen in tapering hairs, broken hairs and black dots whereas for yellow dots to disappear completely in all patches it took 16 weeks. The adverse effects were observed at an earlier stage using dermoscopy than clinically. Conclusion: Intralesional triamcinolone acetonide is efficacious for treatment of localized patchy alopecia areata. Dermoscopy is very useful to identify signs of early clinical response, adverse effects and markers of disease activity.Introduction
Alopecia areata is an autoimmune disease that presents as, well demarcated patches of non scarring hair loss on skin of normal appearance. The exact pathogenesis of the disease yet remains to be clarified; however autoimmune, genetic and environmental factors have been implicated. Alopecia areata occurs in populations worldwide. In the United States, alopecia areata was estimated to occur in 0.1% to 0.2% of the general population, with a lifetime risk of 1.7%. [1] Sixty percent of patients present with their first patch before 20 years of age.
The course of the disease is unpredictable and the response to treatment is variable. The various treatment modalities used can be classified into topical and systemic therapies. The topical therapy includes intralesional corticosteroids, topical corticosteroids, minoxidil, anthralin and topical immunotherapy in the form of diphenylcyclopropenone (DPCP) and squaric acid dibutylester (SADBE). The systemic therapy includes systemic corticosteroids and photochemotherapy. Cyclosporine, methotrexate, sulphasalazine and biologics like etanercept, efalizumab, adalimumab and infliximab have been used with limited success. Intralesional corticosteroids are the treatment of choice for adults with less than 50% of scalp area involvement. [2]
The sensitivity of picking up clinical response to treatment by clinical examination is very variable and has inter individual variation. Dermoscopy is a noninvasive diagnostic tool which visualizes subtle patterns of skin lesions not normally visible to the unaided eye. [3] The characteristic features of alopecia areata on dermoscopy are yellow dots, black dots, broken hair, vellus hair and tapering or exclamation mark hair. [4] After therapy there is a decrease in the number of these characteristic findings. On the other hand, the presence of thin and unpigmented vellus hair within the patch, and evidence of transformation of vellus hair into terminal hair, appearing as increased proximal shaft thickness and pigmentation, is characteristic of remitting disease and indicative of a response to treatment. [5]
The present study was undertaken to evaluate the efficacy of intralesional triamcinolone acetonide in the treatment of alopecia areata and to assess its local and systemic side effects. Dermoscopy was used to identify signs of early clinical response to the chosen therapeutic regimen and also for the early identification of the adverse effects. The useful markers to assess the severity of alopecia areata on dermoscopy are the black dots, yellow dots, broken hairs or dystrophic hair, tapering or exclamation mark hair and short vellus hairs. Previous reports have suggested that the severity of alopecia areata is an important prognostic factor. [4] Therefore, dermoscopic examination of patches of alopecia areata may provide predictors of the response to therapy and can also be utilized for monitoring response to any prescribed regimen.
Methods
This was a hospital based interventional study done in 60 patients with alopecia areata subject to the inclusion and exclusion criteria who attended the dermatology OPD of tertiary care hospital in south India from May 2011 to June 2012, after obtaining clearance from the institute ethics committee (EC/2011/2/5; Dated 25.04.2011) The study group consisted of 60 consenting patients of alopecia areata, satisfying the inclusion and exclusion criteria. The inclusion criteria included alopecia areata of scalp, age >10 years, duration of present patches <1 year, 1-3 patches of alopecia areata, < 50% scalp area involved, patient should not be on any modality of therapy for atleast 2-4 weeks. The exclusion criteria included children <10 years, pregnant women and lactating women, history of steroid allergy, immunocompromised patients, patients having bleeding diathesis, patients having active scalp inflammation, patients receiving systemic steroids, any patient not willing to take part in the study.
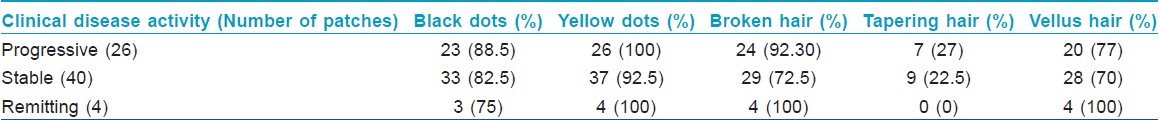
The baseline assessment of alopecia grading was performed using a 6 point scale score called as alopecia grading score (AGS), [6],[7] : S0 = No alopecia, S1 = hair loss <10%, S2 = hair loss 11-25%, S3 = hair loss 26-50%, S4 = hair loss 51-75% and S5 = hair loss >75%. The clinical disease activity [4] of alopecia areata was estimated as follows: Progressive alopecia areata, an increase in total hair loss of more than 5%; stable alopecia areata, a change in total hair loss of less than 5%; remitting alopecia areata, a decrease in total hair loss of more than 5% over the month prior to presentation. The patients fulfilling the diagnosis and criteria (inclusion and exclusion) were enrolled for the study. A baseline digital camera photograph of the patch and dermoscopic parameters at centre of patch and at 3 O′ clock position were recorded which included black dots, yellow dots, vellus hair, broken hair, tapering hair and pigmented hair. The 3 O′ clock position was defined as: For the patch over the forehead to vertex, it was examined from the front (i.e., patient facing the examiner), for the patches in the occipital area patient was examined from behind (i.e., patient showing his back to the examiner) and for the lesions in the temporal area, the patients shoulder faced the examiner while determining the position of the analysis. Heine DELTA 20® dermatoscope was used for the study. The dermoscopic parameter for each patch were counted in the centre of the patch and at one representative field in the periphery designated as 3 O′ clock position and their number documented and a total number for each patch was calculated as summation of readings at centre and periphery.
Triamcinolone acetonide (5 mg/ml) was injected at 1 cm intervals with 0.1 ml on each site. The maximum of 3 ml was injected in each visit using a 0.5 inch long, 30 gauge needle, fitted to an insulin syringe. Injections were repeated every 4 weeks. Patients were followed up at 4 weeks interval and the response was evaluated clinically by using a 5 point semi-quantitative score, regrowth scale (RGS), [6],[7] with the scale ranging from 0 score (regrowth < 10%), 1 score (regrowth 11-25%), 2 score (regrowth 26-50%), 3 score (regrowth 51-75%), 4 score (regrowth ≥ 75%). All sixty patients were given injections of intralesional triamicinolone, repeated once every 4 weeks till the patients achieved an RGS of 4 (regrowth ≥ 75%) and they were evaluated at baseline and at 4 weeks interval for up to a maximum of 24 weeks to record the progress of hair regrowth. The dermoscopic readings at the centre of the patch and at 3 O′ clock position were documented for a maximum of 6 months. [8] The changes in the various dermoscopic parameters were looked for assessing the response to the prescribed therapeutic regimen.
The improvement was recorded using dermoscopy as significant (≥75%), and maximum (100%), change (i.e., reduction in yellow dots, black dots, broken hair and/or tapering hair). The clinical improvement recorded using RGS of 4 scale was regrowth ≥ 75% (maximum clinical improvement). The study also involved identification of side effects clinically and using a dermoscope.
The data collected was tabulated in Microsoft Excel worksheet and computerized analysis was performed using SPSS 17 (SPSS, Chicago, IL, USA). A descriptive statistical analysis was carried out for presenting the dermoscopic parameters and socio-demographical parameters. The outcome variables (clinical response and dermoscopic parameters) were assessed and quantified in each time point (at 4 weeks interval for a maximum follow up period of 6 months). For comparison of means, one way ANOVA was used. Chi-square test (χ2 test) was used to evaluate the association of outcome variables with the socioeconomic and demographic factors. In cases where any one of the cell value was <5, Fischer′s exact test was used. Correlations between the incidence of each dermoscopic finding and the disease activity were analysed using the Spearman rank-order correlation coefficient by rank test. All statistical analyses were carried out at 95% confidence interval and the p value < 0.05 was considered as significant.
Results
A total of 65 patients were recruited in the study. Out of these, 5 patients were lost to follow up and were excluded from the study. In the remaining 60 patients, a total of 70 patches (fifty one patients had one patch, only 8 patients had 2 patches and one patient had 3 patches) were treated with injections of intralesional triamcinolone acetonide (5 mg/ml) at 4 weeks interval and they were analysed for therapeutic response. [Table - 1] shows the main demographic and clinical characteristics of enrolled patients. At baseline the alopecia grading score was 1.34 (ranging from 1-3 with a median of 1 and a SD of ± 0.56).

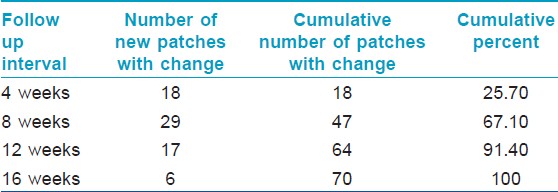
All sixty patients were given injections of intralesional triamicinolone, for a maximum of 6 months after baseline evaluation and the treatment was repeated at 4 weeks interval till the patients achieved an RGS of 4 (regrowth ≥ 75%). The regrowth score at 4 weeks interval is as given in [Table - 2].
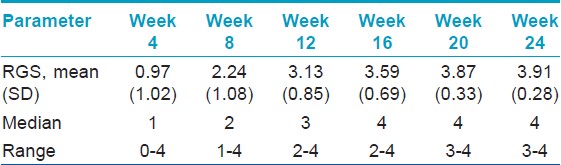
At 4 th week, 3 patients showed nearly complete recovery with a RGS of 4 and hence received only a single intralesional triamicinolone injection and completed the study. At 8 th week, 10 more patients showed nearly complete recovery with a RGS of 4 and hence received 2 intralesional triamicinolone injections and completed the study. At 12 th week, 15 more patients showed nearly complete recovery with a RGS of 4 and hence received 3 intralesional triamicinolone injections and completed the study. At 16 th week, 17 more patients showed nearly complete recovery with a RGS of 4 and hence received 4 intralesional triamicinolone injections and completed the study. At 20 th week, 9 more patients showed nearly complete recovery with a RGS of 4 and hence received 5 intralesional triamicinolone injections and completed the study. At 24 th week, 3 more patients showed nearly complete recovery with a RGS of 4 and hence received 6 intralesional triamicinolone injections and completed the study. Result of [Table - 3] summarizes the maximum clinical improvement (RGS of 4) in alopecia areata patients with the duration of treatment.
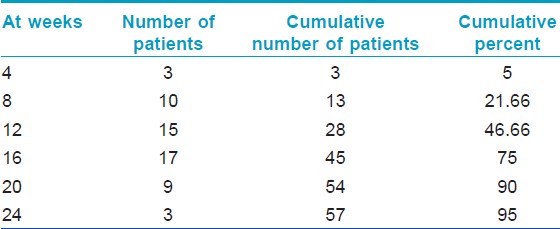
At the end of 24 weeks of follow up, there were 3 patients (5%) who did not achieve a RGS of 4. There were no subsequent intralesional triamcinolone acetonide injections given to any patient. One of these was started on tretinoin and tacrolimus and achieved an RGS of 4 after 30 weeks. Another patient was started on minoxidil by an outside practitioner and achieved RGS of 4 (regrowth ≥ 75%) after 36 weeks. The last of these three patients who did not achieve an RGS of 4, was not started on any other treatment and achieved an RGS of 4 after 36 weeks.
Based on the clinical response the patients were categorized into early responders-28 cases (those achieving RGS of 4 within 12 weeks of therapy), late responders-29 cases (those achieved RGS of 4 between 12-24 weeks of therapy) and incomplete responders 3 cases (who did not achieve a RGS of 4 at 24 weeks following initiation of therapy). A statistically significant association was seen in the early responders with the duration of patch < 1 month (p0 = 0.0360) and size of patch < 4 cm (p = 0.0002). Late and incomplete responders showed a statistically significant association of their disease with a family history of alopecia areata (p0 < 0.0001), presence of recurrent disease (p = 0.0147) and presence of nail changes ( p = 0.0007). There was no statistically significant association of the clinical response seen with the age of the patient, the age of onset of disease, gender, presence of associated systemic disorder like atopy, family history of any other disease and loss of eyebrows.
Yellow dots were the predominant dermoscopic finding which was seen in 95% of the patches followed by black dots (84%), broken hair (81%) and vellus hair (74%). Tapering hair was seen in 23% of the patches. Result of [Table - 4] summarizes the dermoscopic findings at baseline in the 70 patches studied. Fifty three patches (88.3%) had ≥3 dermoscopic findings at baseline that included any combination of black dots, yellow dots, broken hair, tapering hair and/or vellus hair. Coudability hairs were seen in 8 out of 70 patches at baseline (11%) in which 5 patches had 1-3 coudability hairs (score 1), 3 patches had 4-9 coudability hairs (score 2). Pseuodomonilethrix was seen in 2 out of 70 patches at baseline (3%).
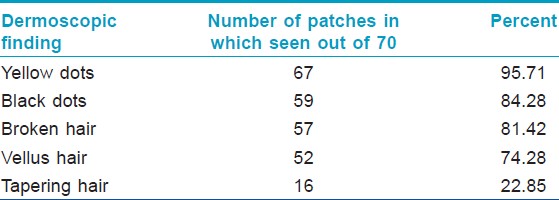
The effect of treatment on hair regrowth in alopecia areata was apparent in the centre of the patch in contrast to the periphery in the early part of the treatment. At 4 weeks, 60 patches demonstrated regrowth of new vellus hairs, subsequently at 8 weeks, 7 more patches and at 12 weeks, 3 more patches had regrowth with vellus hairs [Figure - 1], [Figure - 2], [Figure - 3]. Occurrence of atleast a single pigmented hair after treatment showed that at 4 weeks, 40 patches demonstrated atleast a single pigmented hair, subsequently 25 more patches had this at 8 weeks and by 12 weeks, 5 more patches demonstrated this dermoscopic change.
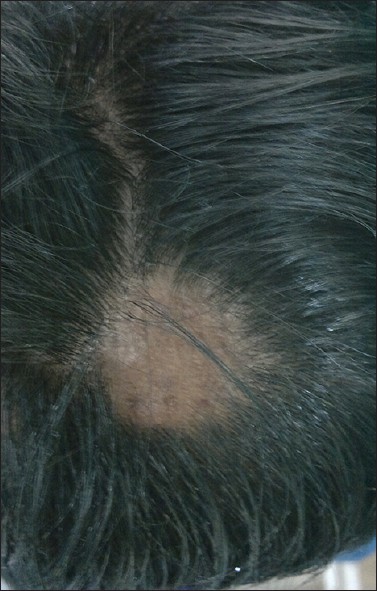 |
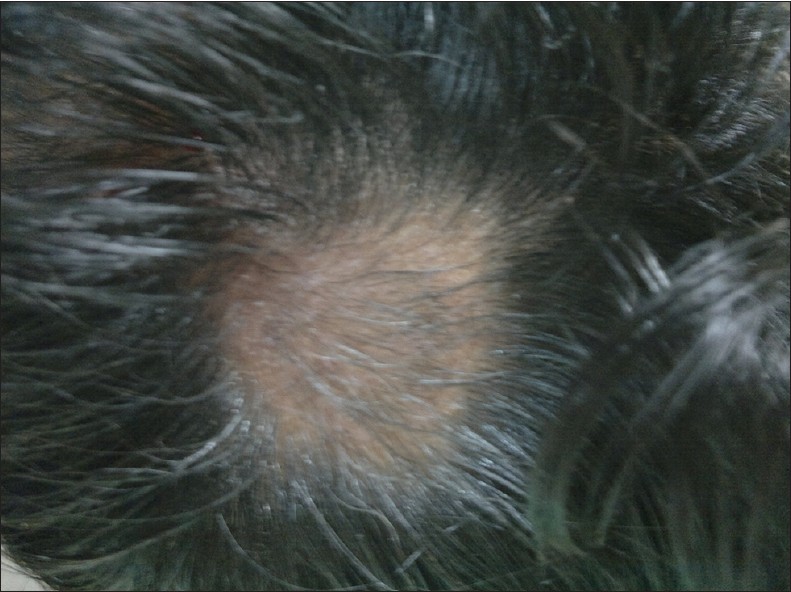
| Figure 1: Baseline photograph showing smooth bald oval patch over the occipital area of scalp |
 |
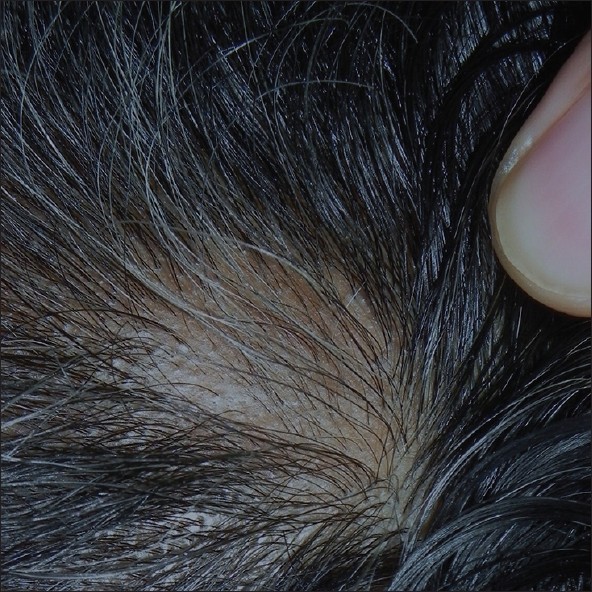
| Figure 2: At 8 weeks, after 2 intralesional steroids injections showing 50-75% regrowth of terminal hair |
 |
| Figure 3: At 16 weeks, after 4 intralesional injections showing most of hair regrowth in form of terminal hair |
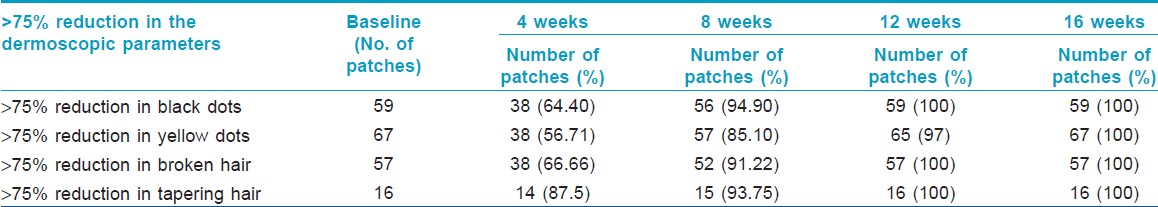
A comparison of the time taken to achieve significant change in dermoscopic parameters from baseline amongst black dots, yellow dots, broken hair and tapering hair in the 70 patches studied showed the findings as per [Table - 5]. This table shows that amongst the four parameters studied, the tapering hair finding reduced maximally at 4 weeks (87.5%), followed by broken hair and black dots in the 70 patches. The yellow dots responded least (56.7%) to the treatment amongst the four parameters. At 8 weeks, there is a nearly equal response in tapering hairs, broken hairs and black dots finding in the 70 patches studied. At 8 weeks also the yellow dots were least responsive to the treatment amongst the four parameters (85.10%). At 12 weeks, complete disappearance was seen in tapering hairs, broken hairs and black dots finding in all the 70 patches studied whereas, for yellow dots to disappear completely in all the 70 patches it took 16 weeks [Figure - 4], [Figure - 5], [Figure - 6], [Figure - 7], [Figure - 8].
 |
| Figure 4: Baseline dermoscopic photograph showing yellow dots, black dots, broken hair in the centre of the patch |
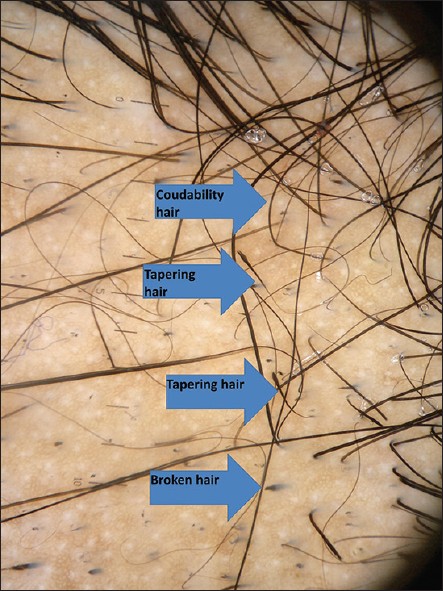 |
| Figure 5: Baseline dermoscopic photograph showing tapering hairs and coudability hairs in the periphery of the patch |
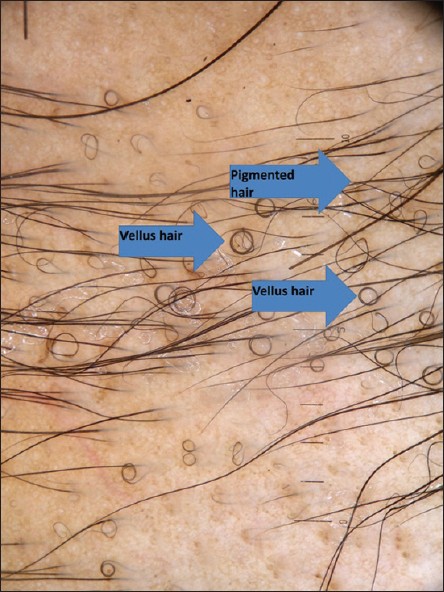 |
| Figure 6: At 4 weeks dermoscopy showing regrowth of vellus hair as circular hairs and few pigmented hairs |
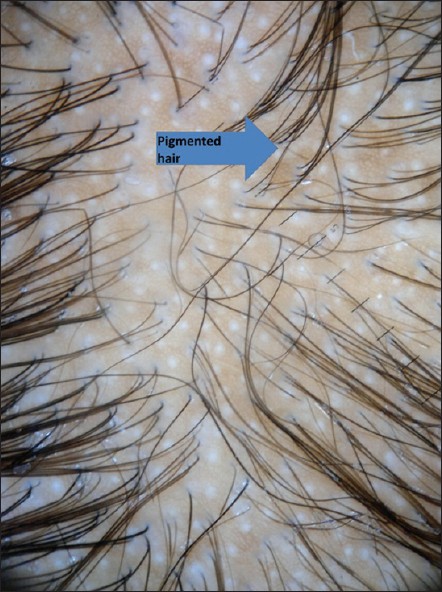 |
| Figure 7: At 12 weeks dermoscopy showing regrowth with numerous pigmented hair and few yellow dots and complete disappearance of broken hair, black dots and tapering hairs |
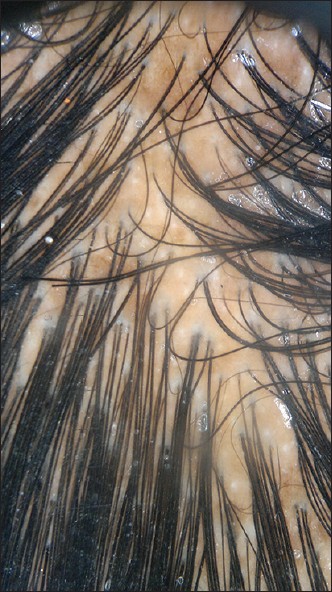 |
| Figure 8: At 16 weeks dermoscopy showing pigmented hairs covering almost whole of the patch |
The clinical disease activity was evaluated in each patient and classified as remitting, stable or progressive. The dermoscopic features seen at baseline in patients with the indicated disease activity of alopecia areata are shown as in [Table - 6]. Correlation of the dermoscopic findings with the disease activity of alopecia areata was not statistically significant using the Spearman rank-order correlation coefficient by rank test.
Maximum dermoscopic change was defined as 100% reduction from baseline in all the following dermoscopic parameters i.e., black dots, yellow dots, broken hair and tapering hair and showed that 67% (47) of the patches showed maximum dermoscopic change by 8 weeks and 91% (64) of the patches showed maximum dermoscopic change by 12 weeks and 100% (70) of the patches showed maximum dermoscopic change by 16 weeks. The findings are as enumerated in [Table - 7]. The comparison between the dermoscopy and clinical examination in detecting maximum improvement at different follow up periods is as given in [Table - 8] and [Figure - 9]. Thus the sensitivity to pick up an early response by dermoscopy is significantly higher as compared to clinical examination at 4 th week, 8 th week, 12 th week and 16 th week ( P < 0.05, χ2 test).
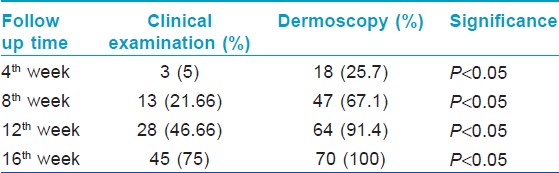
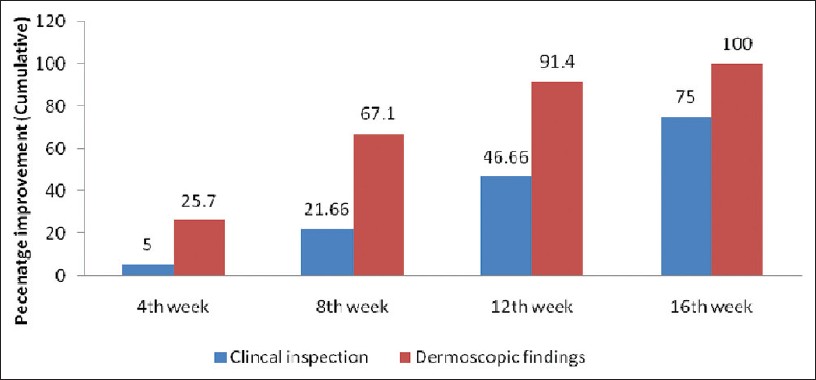 |
| Figure 9: Maximum improvement (cumulative percentage) detected using dermoscopy and on clinical examination in alopecia areata patients |
Atrophy was seen in 11 patches (16%) and telangiectasia in 2 patches (3%) dermoscopically. Fifty seven patches (81.4%) did not show any adverse effects. At 4 weeks 7% of the patches showed adverse effects like atrophy and telangiectasia to intralesional triamcinolone acetonide. This increased to 17% of patches showing these adverse effects to intralesional triamcinolone acetonide at 8 weeks and to 18.50% of patches by 12 weeks. This shows that if the adverse effects had to occur in a particular patch, it occurred by 12 weeks and no significant association was found between the number of injections given and the development of the adverse effects. The adverse effects were observed at an earlier stage by using dermoscopy and thus the area of the patch identified dermoscopically, showing atrophy and/or telangiectasia was not reinjected. No systemic side effects were observed. There were 3 patients (5%) who developed clinically apparent atrophy and these were shifted to a new treatment regimen, of whom 1 patient each received tretinoin, tacrolimus and minoxidil to manage steroid induced atrophy. This adverse effect occurred in each of the 3 patients at 16 weeks and at that time they had achieved a RGS of 4 (regrowth ≥ 75%) and thus no further injections were given to them.
Discussion
Alopecia areata is a disease characterized by non-scarring hair loss on the scalp or any hair-bearing surface. A wide range of clinical presentations can occur, from a single patch of hair loss to complete loss of hair on the scalp (alopecia totalis) or the entire body (alopecia universalis). Direct and indirect evidence supports an autoimmune aetiology for alopecia areata. T-lymphocytes are predominantly present in the peribulbar inflammatory infiltrate and are presumed to play a role in hair loss. Alopecia areata frequently occurs in association with other autoimmune diseases, such as thyroiditis and vitiligo. Autoantibodies to follicular components have been detected. [5] Various therapeutic agents have been developed for the treatment of alopecia areata, but none is curative or preventive. [2] The aim of alopecia areata treatment is to suppress the activity of disease. The various treatment modalities can be classified into topical and systemic therapies. The topical therapy includes intralesional corticosteroid, topical corticosteroid, minoxidil, anthralin and topical immunotherapy in the form of DPCP and SADBE. The systemic therapy includes systemic corticosteroids and photochemotherapy. Cyclosporine, methotrexate, sulphasalazine and biologics have been used with limited success. Spontaneous remission occurs in up to 80% of patients with limited patchy hair loss of short duration (<1 year). [9]
Previous studies are mostly on alopecia areata of subtotal to universal type. [10],[11],[12] In our study, we chose patients with minimal scalp surface area involvement mainly to assess the response of the patches clinically and dermoscopically following intralesional triamcinolone acetonide injection at 4 weeks interval. Thus our study population included 49 cases (85%) with hair loss < 10% of scalp area. There were 10 cases (13.3%) with hair loss 11-25% of scalp area. Only a single patient (1.7%) had hair loss > 25% of scalp area.
An Indian study, [13] showed that 4% of the patients achieved a clinical regrowth >75% at 3 weeks following intralesional triamcinolone acetonide injection at 3 weeks interval which is similar to our study showing 5% of patients achieving a clinical regrowth > 75% at 4 weeks following intralesional triamcinolone acetonide injection at 4 weeks interval. In various studies, [10],[13] nearly complete hair regrowth (>75%) following treatment with intralesional triamcinolone acetonide has been shown to be in 60-71% of patients at 12 weeks follow up. Our study showed nearly complete hair regrowth (>75%) in 47% of patients at 12 weeks.
Concentrations of triamcinolone acetonide ranging from 5-10 mg/ml have been used in various studies, [10],[13],[14] and in our study, we used 5 mg/ml, mainly to minimize the adverse effects to the intralesional triamcinolone acetonide. The dosing regimen employed in various studies has ranged from a single injection at baseline, [11],[14] to a total of 3 injections every 2 weeks, [10] to once in 3 weeks for 12 weeks. [13] We gave injections once in 4 weeks for a maximum of 6 months depending on the response achieved. If no improvement occurred after 6 months, then the treatment with intralesional triamcinolone acetonide was stopped. The reduced efficacy in some patients is postulated to be due to a decreased expression of thioredoxin reductase 1 in the outer root sheath of the hair follicles. [8]
The follow up period for observation in most studies, [10],[13],[15] has ranged from 12-16 weeks for evaluation of therapeutic efficacy to intralesional triamcinolone acetonide whereas in our study it was 24 weeks, which allowed for a longer time period for evaluation of the therapeutic response and hence we observed a higher percentage of response at the completion of study of about 95%. This fact has been previously substantiated in a previous study, [14] which reported that the effect of a single injection of triamcinolone acetonide can persist for at least 9 months. Thus it is imperative to allow for a longer period of observation to assess the response to treatment.
Our study showed a higher percentage of response as compared to previous studies and this can be attributed to a number of factors. Firstly, the surface area of scalp involved in the majority of patients, 49 cases (85%) was < 10% (AGA score of S1). Only a single patient (1.7%) had hair loss > 25% of scalp area. Secondly, the observation period in our study was 24 weeks, which is substantially higher than that of other studies, thus allowing for a longer time for evaluation of therapeutic efficacy to intralesional triamcinolone acetonide. Thirdly, injections of intralesional triamcinolone acetonide were given up to a maximum of 6 months at 4 weeks interval, depending on the response achieved thus a longer duration of therapy was given to the patients as compared to other studies.
The poor prognostic factors, [16] associated with alopecia areata include an early age of onset, extensive scalp involvement (>50% scalp area), loss of eyebrows and eyelashes, alopecia totalis or universalis, recurrent episodes, ophiasis, sisaipho and reticular pattern of alopecia, nail changes that include pitting, onychodystrophy, onycholysis, anonychia, presence of associated systemic disorders like atopy, hypertension and connective tissue disease and a family history of alopecia areata. Another study, [15] has stated that regrowth was more likely in patients with few lesions (less than 5 patches), lesions of short durations (<1 month) and patches < 3 cm in diameter. In our study, we found that patients with duration of patch < 1 month and size of patch < 4 cm responded early to treatment. The cases with family history of alopecia areata, history of recurrent disease and nail changes had a late and/or an incomplete response.
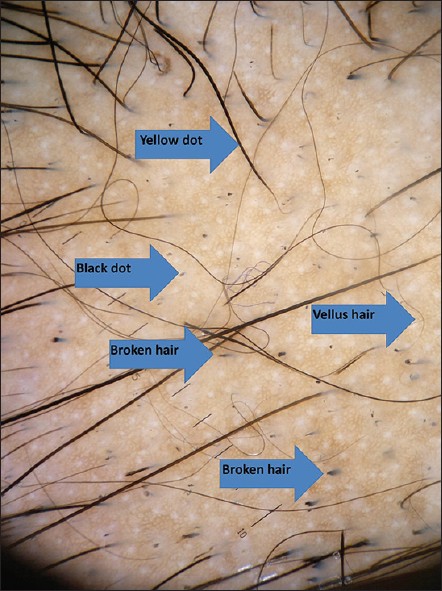
Dermoscopy is a noninvasive diagnostic tool which visualizes subtle patterns of skin lesions not normally visible to the unaided eye. Dermoscopy has recently become a useful diagnostic tool for alopecia areata, especially in doubtful cases. Yellow dots, black dots, broken hairs, tapering hairs and short vellus hairs are characteristic dermoscopic features in alopecia areata. [4] Yellow dots are the most sensitive feature for the diagnosis of alopecia areata. [4],[17] They are due to dilatation of the affected follicular infundibulum with keratinous material or sebum. [4] Black dots are remnants of exclamation hair and broken hair. They represent pigmented hairs broken or destroyed at the scalp level. They provide a sensitive marker of disease activity and disease severity. [4] Broken hairs are hair with fractured tips. They may be fractured before emergence from the scalp known as cadaverized hair, or may appear as short twisted dystrophic hairs. They are also considered to be clinical markers of disease activity and severity. [4] Tapering or exclamation mark hair result from a truncated hair cycle consisting of premature telogen and dystrophic anagen and hence are fractured and short. These are indicative of an active disease process and are more commonly seen in the periphery of the lesion. Vellus hair results from hair regrowth either spontaneously or as a result of treatment. They can be seen by the dermoscope even when they are hardly perceived by the naked eye and hence shows the non-scarring nature of alopecia areata. Not only the pigmented short vellus hairs can be detected by dermoscopy, but also the white vellus hairs are easily seen. This shows the negative correlation of vellus hairs with the disease activity or severity of alopecia areata. This finding on follow up visits can be used to encourage patients to continue treatment. The pigmented skin of Asian patients helps in easy detection of vellus hairs. [4] Although yellow dots are seen in androgenetic alopecia, female androgenetic alopecia, and trichotillomania, the number of yellow dots is limited in these conditions relative to alopecia areata, which shows numerous yellow dots. In addition, black dots, tapering hairs, and broken hairs are specific for alopecia areata, except for trichotillomania, which can be diagnosed by the number of yellow dots, black dots, and the lack of tapering hairs. [4]
The regrowth of short vellus hairs can be seen after treatment, including steroid pulse therapy even when the recovered hairs are hardly perceived by the naked eye. [18] It has been previously recorded that with treatment, when the initial regrowth occurs, it may or may not be clinically detectable as new, thin and unpigmented hairs within the patch. The presence of these vellus hairs and evidence of transformation of vellus hair into terminal hair, appearing as increased proximal hair shaft thickness and pigmentation, are characteristic of remitting disease and hence indicators of a response to treatment. [5]
At 12 weeks, complete disappearance was seen in tapering hairs, black dots and broken hairs in all the 70 patches studied whereas for yellow dots to disappear completely in all the 70 patches, it took 16 weeks. Thus it can be indirectly inferred that tapering hairs, broken hair and black dots in that order, are markers of disease activity in alopecia areata as had been shown by Inui et al., [4] and are the first parameters to respond to treatment and thus imply a subsidence of disease activity with treatment. Yellow dots being the least responsive amongst them, also goes in accordance with the study by Inui et al., [4] which had shown no statistically significant association of disease activity with yellow dots.
Adverse effects of therapy with intralesional triamcinolone acetonide include transient atrophy and telangiectasia. [13] Anaphylactic reaction to intralesional corticosteroid injection has been reported due to carboxymethylcellulose, a dispersant in corticosteroid preparation and also to triamcinolone acetonide per se. [19] A study, [13] from India reported atrophy in 23% of treated patients seen clinically. In our study, the adverse effects observed using dermoscopy were atrophy in 11 patches (16%) and telangiectasia in 2 patches (3%). No systemic side effects were observed in our study. There were 3 patients (5%) who developed clinically apparent atrophy in our study. The reduced percentage of clinically apparent atrophy seen in our study is because area of the patch identified dermoscopically, showing atrophy and/or telangiectasia was not reinjected hence avoiding the development of clinically identifiable atrophy.
To conclude, intralesional steroids remain the first choice for the treatment of localized patchy alopecia areata. A longer follow up period is advisable to observe a greater response to therapy. Dermoscopy has recently become a useful tool for the diagnosis and management of alopecia areata. Our study has demonstrated the usefulness of dermoscopy to identify signs of early clinical response to intralesional triamcinolone acetonide. The sensitivity to pick up an early response by dermoscopy is significantly higher as compared to clinical examination. Moreover dermoscopy, helps to identify markers of disease activity. A lower concentration of intralesional triamcinolone is advisable to minimise the adverse effects and it also maintains the efficacy of therapy at the same time. Dermoscopy can be used to identify areas showing early atrophy and hence subsequent injections in the same site can be avoided thus preventing clinically apparent atrophy.
| 1. |
Safavi K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch Dermatol 1992;128:702.
[Google Scholar]
|
| 2. |
Alkhalifah A, Alsantali A, Wang E, McElwee KJ, Shapiro J. Alopecia areata update: Part II. treatment. J Am Acad Dermatol 2010;62:191-202.
[Google Scholar]
|
| 3. |
Nischal KC, Khopkar U. Dermoscope. Indian J Dermatol Venereol Leprol 2005;71:300-3.
[Google Scholar]
|
| 4. |
Inui S, Nakajima T, Nakagawa K, Itami S. Clinical significance of dermoscopy in alopecia areata: Analysis of 300 cases. Int J Dermatol 2008;47:688-93.
[Google Scholar]
|
| 5. |
Lacarrubba F, D'Amico V, Nasca MR, Dinotta F, Micali G. Use of dermatoscopy and videodermatoscopy in therapeutic follow-up: A review. Int J Dermatol 2010;49:866-73.
[Google Scholar]
|
| 6. |
Tosti A, Iorizzo M, Botta GL, Milani M. Efficacy and safety of a new clobetasol propionate 0.05% foam in alopecia areata: A randomized, double-blind placebo-controlled trial. J Eur Acad Dermatol Venereol 2006;20:1243-7.
[Google Scholar]
|
| 7. |
Mancuso G, Balducci A, Casadio C, Farina P, Staffa M, Valenti L, et al. Efficacy of betamethasone valerate foam formulation in comparison with betamethasone dipropionate lotion in the treatment of mild-to-moderate alopecia areata: A multicenter, prospective, randomized, controlled, investigator-blinded trial. Int J Dermatol 2003;42:572-5.
[Google Scholar]
|
| 8. |
Sohn KC, Jang S, Choi DK, Lee YS, Yoon TJ, Jeon EK, et al. Effect of thioredoxin reductase 1 on glucocorticoid receptor activity in human outer root sheath cells. Biochem Biophys Res Commun 2007;356:810-5.
[Google Scholar]
|
| 9. |
Ikeda T. A new classification of alopecia areata. Dermatologica 1965;131:421-45.
[Google Scholar]
|
| 10. |
Abell E, Munro DD. Intralesional treatment of alopecia areata with triamcinolone acetonide by jet injector. Br J Dermatol 1973;88:55-9.
[Google Scholar]
|
| 11. |
Ferrando J, Moreno-Arias GA. Multi-injection plate for intralesional corticosteroid treatment of patchy alopecia areata. Dermatol Surg 2000;26:690-1.
[Google Scholar]
|
| 12. |
Chang KH, Rojhirunsakool S, Goldberg LJ. Treatment of severe alopecia areata with intralesional steroid injections. J Drugs Dermatol 2009;8:909-12.
[Google Scholar]
|
| 13. |
Kuldeep C, Singhal H, Khare AK, Mittal A, Gupta LK, Garg A. Randomized comparison of topical betamethasone valerate foam, intralesional triamcinolone acetonide and tacrolimus ointment in management of localized alopecia areata. Int J Trichology 2011;3:20-4.
[Google Scholar]
|
| 14. |
Porter D, Burton JL. A comparison of intra-lesional triamcinolone hexacetonide and triamcinolone acetonide in alopecia areata. Br J Dermatol 1971;85:272-3.
[Google Scholar]
|
| 15. |
Kubeyinje EP. Intralesional triamcinolone acetonide in alopecia areata amongst 62 Saudi Arabs. East Afr Med J 1994;71:674-5.
[Google Scholar]
|
| 16. |
Messenger AG. Alopecia areata. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. 8 th ed. Oxford: Blackwell Science Ltd; 2010. p 66.31-66.38.
th ed. Oxford: Blackwell Science Ltd; 2010. p 66.31-66.38.'>[Google Scholar]
|
| 17. |
Ross EK, Vincenzi C, Tosti A. Videodermoscopy in the evaluation of hair and scalp disorders. J Am Acad Dermatol 2006;55:799-806.
[Google Scholar]
|
| 18. |
Nakajima T, Inui S, Itami S. Pulse corticosteroid therapy for alopecia areata: Study of 139 patients. Dermatology 2007;215:320-4.
[Google Scholar]
|
| 19. |
De Souza BA, Bantick G. Anaphylactic reaction to intralesional steroid injection. Plast Reconstr Surg 2006;117:336.
[Google Scholar]
|
Fulltext Views
5,949
PDF downloads
1,629





