Translate this page into:
Efficacy and safety of a nano-emulsion gel formulation of adapalene 0.1% and clindamycin 1% combination in acne vulgaris: A randomized, open label, active-controlled, multicentric, phase IV clinical trial
2 Dr. Amiya Mukhopadhyay's Skin Clinic, Asansol, India
3 Clinical Research, Cadila Healthcare Ltd., Ahmedabad, India
4 Dr. Kelkar's Skin Clinic, Pune, India
5 Twacha Skin Care and Laser Centre, Navsari, India
6 Swarnkar Skin Centre, Indore, India
7 Bajaj Medicine and Skin Clinic, Ludhiana, India
8 R.S.V. Skin Clinic, Chennai, India
9 NDDS, Zydus Research Centre, Cadila Healthcare Ltd., Ahmedabad, India
Correspondence Address:
Amit Kubavat
Cadila Healthcare Ltd., Zydus Tower, 9th Floor, Opp. ISKCON Temple, Satellite Cross Roads, Ahmedabad - 380015, Gujarat
India
| How to cite this article: Prasad S, Mukhopadhyay A, Kubavat A, Kelkar A, Modi A, Swarnkar B, Bajaj B, Vedamurthy M, Sheikh S, Mittal R. Efficacy and safety of a nano-emulsion gel formulation of adapalene 0.1% and clindamycin 1% combination in acne vulgaris: A randomized, open label, active-controlled, multicentric, phase IV clinical trial. Indian J Dermatol Venereol Leprol 2012;78:459-467 |
Abstract
Background: Acne vulgaris is a very common skin disease with a significant detrimental effect on the quality of life of the patients. Aims: To assess the comparative efficacy and safety of a nano-emulsion gel formulation of adapalene and clindamycin combination with its conventional formulation in the treatment of acne vulgaris of the face. It was a prospective, randomized, open label, active-controlled, multicentric, clinical trial. Methods: Eligible patients suffering from acne vulgaris of the face were randomized to receive once-daily treatment with a nano-emulsion gel or conventional gel formulation of adapalene 0.1% and clindamycin (as phosphate) 1% combination for 12 weeks. Total, inflammatory and noninflammatory lesion counts, with grading of acne severity were carried out on a monthly basis. Safety assessments were done to determine the comparative local and systemic tolerability. Two-tailed significance testing was carried out with appropriate statistical tests, and P-values < 0.05 were considered as significant. Results: 209/212 patients enrolled in the study were eligible for efficacy and safety assessments in both nano-emulsion gel (118/119 patients) and conventional gel (91/93 patients) groups. Significantly better reductions in total (79.7% vs. 62.7%), inflammatory (88.7% vs. 71.4%) and noninflammatory (74.9% vs. 58.4%) lesions were reported with the nano-emulsion gel as compared to the conventional gel (P < 0.001 for all). Mean acne severity score also reduced significantly more with the nano-emulsion formulation (1.9 ± 0.9 vs. 1.4 ± 1.0; P < 0.001) than the comparator. Significantly lower incidence and lesser intensity of adverse events like local irritation (4.2% vs. 19.8%; P < 0.05) and erythema (0.8% vs. 9.9%; P < 0.05) were recorded with the nano-emulsion gel. Conclusions: The nano-emulsion gel formulation of adapalene and clindamycin combination appears to be more efficacious and better tolerated than the conventional formulation for the treatment of acne vulgaris in Indian patients. Further studies can elucidate the comparative treatment benefits of this nano-emulsion gel formulation.Introduction
Acne vulgaris is a very common skin disease, which causes a high degree of psychosocial suffering [1] and has a detrimental effect on the quality of life of the patients irrespective of age or gender. [2],[3],[4] Acne vulgaris of the face is reported to be the commonest presentation by an Indian tertiary care centre; where ~1% of all dermatology patients of outpatient department suffered from acne vulgaris, irrespective of the site of the lesions. [5]
Pathophysiology of acne primarily includes a complex interaction between the presence and activity of Propionibacterium acnes, inflammation and hyperkeratinization. [6] Treatment of acne is principally directed towards these known pathogenic factors. Combination therapy with a topical retinoid and an antimicrobial agent, which addresses majority of the causative factors of acne, is considered a first-line treatment option for almost all patients. [7] Adapalene is a third generation, synthetic retinoid compound with generally better tolerability in its therapeutic class, including novel microsphere formulation of tretinoin. [8],[9],[10] Clindamycin and erythromycin are the commonly prescribed topical antibiotics for acne vulgaris with anti-inflammatory properties, among which the efficacy of clindamycin has remained better over a period of time. [11],[12] Adapalene is also shown to increase follicular penetration of clindamycin, [13] and their combination therapy is reported to be highly efficacious and well-tolerated. [14],[15]
A combination preparation of adapalene 0.1% w/w and clindamycin (as phosphate) 1% w/w has been developed using the nano-emulsion technology. It is designed to deliver the active ingredients with a good penetration into the pilo-sebaceous glands to provide better efficacy with good tolerability. Further, the preparation uses an aqueous-based gel vehicle with moisturizing properties to enhance tolerability and is available as a once-daily gel formulation.
The present study was conducted to assess the efficacy and safety of therapy with this nano-emulsion gel formulation of adapalene 0.1% and clindamycin 1% combination as compared to its conventional gel formulation in patients suffering from acne vulgaris of the face.
Methods
Subjects
This prospective, randomized, open label, active-controlled, multicentric, postmarketing, clinical study was undertaken from 1 st October, 2010 to 23 rd May 2011. Patients of either gender of at least 12 years of age with an established diagnosis of acne vulgaris of the face, who were likely to be available for all visits during the follow-up period and willing to sign an informed consent, were enrolled in the study. Female patients were required not to be pregnant or lactating at the time of enrolment and not planning pregnancy during the study period.
Patients with a history of regional enteritis, ulcerative colitis or antibiotic-associated colitis, significant cardiovascular, hepatic, renal or any other systemic illnesses were not eligible for enrolment. Patients with an open or incompletely-healed wound at the affected site were excluded from the study. Patients with hypersensitivity to preparations containing clindamycin, lincomycin, adapalene, other retinoids or any other related class of the compounds were excluded from the study. Patients having participated in any other clinical trial in the preceding 3 months or with a continuing history of alcohol and/or drug abuse were also not eligible for enrolment.
Patients were not permitted to take any other systemic or topical treatment for acne vulgaris concomitantly during the course of the study. Concomitant use of peeling agents, abrasive cleansers, strong drying agents, astringents or irritant products (with aromatic and alcoholic agents) was also not permitted. Concomitant use of comedogenic cosmetics that can exacerbate acne lesions was strictly avoided. Use of medications with neuromuscular blocking properties was also not permissible during the entire study period.
Procedures
This clinical study was approved by an Independent Ethics Committee (EC) for each of the 7 participating study centers. The study was conducted in compliance with the good clinical practice guidelines issued by International Conference on Harmonisation (ICH-GCP) and the ethical principles of Declaration of Helsinki. Written informed consent was obtained from each of the participating patients.
Patients suffering from acne vulgaris of the face were evaluated as per the inclusion and exclusion criteria and underwent a thorough general physical and systemic examination to assess eligibility for enrolment before the initiation of the treatment. Eligible patients were randomized to receive either adapalene 0.1% and clindamycin (as phosphate) 1% nano-emulsion gel formulation (Adalene® Nanogel TM , Cadila Healthcare Ltd., India) or a marketed conventional gel formulation (Deriva-CMS® Gel, Glenmark Pharmaceuticals Ltd., India), according to a centralized computer-generated randomization schedule.
Patients were instructed to wash the face with a gentle skin cleanser twice daily, rinse immediately and pat the skin dry with a towel. A thin film of either gel was to be applied on the acne-affected areas once a day after washing and before retiring at night, with the fingertips, avoiding the eyes and lips and to leave the treated areas undisturbed overnight. Patients were further instructed to avoid/minimize sun exposure and curtail use of cosmetics during the day. The total treatment duration was 12 weeks in each of the study groups. The enrolled patients were followed-up on an outpatient basis with scheduled visits at weeks 4, 8 and 12 after the initiation of therapy.
The efficacy assessments were carried out by recording the number of noninflammatory lesions (open and closed comedones), inflammatory lesions (papules, pustules, nodules and cysts) and total lesions. Acne severity was assessed by the acne severity grades / scores mentioned in [Table - 1], which has been previously used by other investigators. [16],[17] Treatment success was defined as the attainment of "clear" or "almost clear" grades of acne severity score at the end of treatment phase.

Adverse events were documented by the investigators on each of the scheduled visits, including date of onset and end (duration), intensity (mild, moderate or severe), treatment required and outcome. The relationship of the study medication to each adverse event was determined by World Health Organization-Uppsala Monitoring Centre (WHO-UMC) criteria. The overall assessment of tolerability was given to each of the study medication on a 4-point rating scale at the end of the study by the investigators.
Statistical analysis
Intention to treat (ITT) efficacy and safety assessments were carried out comprising of all the enrolled patients who had received treatment with the study medication and had undergone at least 1 post-baseline assessment. Last observation carried forward (LOCF) procedures were followed for missing data values. Primary efficacy variables were % improvement in total, inflammatory and noninflammatory lesions at the end of therapy (i.e., week 12) and at each follow-up visit as compared to baseline (i.e., week 0). Secondary efficacy variables were the treatment success rate and the degree of improvement in the acne severity score at the end of therapy (i.e., week 12) and at each follow-up visit as compared to baseline (i.e., week 0). Efficacy data is presented as mean, standard deviation (SD), standard error of mean (SE) and 95% confidence intervals (CI) for continuous variables (as specified) and frequency (number) and % of patients with each severity grade / score of acne. Statistical analysis was carried out using T-test, Chi-square Test and Fischer′s Exact Test according to the data characteristics and distributions. P-values<0.05 for 2-tailed assessments were considered as statistically significant.
Results
Patients′ disposition
A total of 212 patients at 7 different centers, spread all across the country, suffering from acne vulgaris of the face were enrolled in the study; out of these, 119 patients were randomized to the nano-emulsion gel arm (group N) while 93 patients were randomized to the conventional gel arm (group C). ITT population comprised of 118 patients in group N and 91 patients in group C who had undergone at least 1 postbaseline efficacy and safety assessment. Flow of the patients enrolled in the study is shown in [Figure - 1].
 |
| Figure 1: Flow of the patients enrolled in the study, N = Nano-emulsion gel, C = Conventional gel |
Demographic and baseline characteristics
The detailed demographic profile and baseline disease characteristics of the ITT population are shown in [Table - 2]. There were similar proportions of male and female patients enrolled in each of the study groups (P = 0.710). Both the treatment groups were comparable for the demographic characteristics, except for age (P = 0.004). Baseline disease characteristics were comparable in both the groups with a similar mean number of total, inflammatory and noninflammatory lesions in each group (P = 0.283, 0.402 and 0.418, respectively). At baseline, majority of the patients had acne severity grade of 2, 3 or 4. The acne severity grades were also not significantly different among the 2 treatment groups at baseline (P = 0.158).

Efficacy analysis
In all the enrolled patients in each of the treatment groups, acne lesions reduced during the course of study after the initiation of therapy. Significantly greater mean % reductions in total, inflammatory and noninflammatory lesions (P < 0.001 for all) were reported in group N as compared to group C at week 12 as is also shown in [Table - 3]. Further, mean % reductions in total, inflammatory and noninflammatory lesions at each follow-up visit as compared to baseline (i.e., week 0) are shown in [Figure - 2]. The reductions in total, inflammatory as well as noninflammatory lesions were statistically significantly more in group N than in group C as early as week 4 and remained significantly better throughout the course of assessments thereafter. Treatment success rate as defined earlier was 58.5% [49.6% - 67.4%] in group N while it was 26.4% [17.3% - 35.4%] in group C (P < 0.001). Mean acne severity score decreased by 1.9 ± 0.9 [1.71 - 2.02] in group N while the reduction observed in group C was 1.4 ± 1.0 [1.17 - 1.58] (P < 0.001). The change in acne severity grades reported in the patients during the course of the study in each of the treatment groups is shown in [Figure - 3]. Thus, the overall acne severity was significantly reduced in group N as compared with group C as early as after 4 weeks of therapy and persisted during the entire course of the study thereafter (P < 0.05 for all). Further, it was reported that 1 patient each in group N (after week 8) and group C (after week 4) discontinued from the study due to lack of efficacy. [Figure - 4] shows the change in acne lesions as observed at baseline and at the end of treatment in a selected patient in group N.
 |
| Figure 2: The mean % reductions in total, inflammatory and noninflammatory lesions during the course of study as compared to baseline, N = Nano-emulsion gel, C = Conventional gel, I-bars = SE (Standard Error of Mean), Group N: N = 118, Group C: N = 91, *P < 0.05, †P < 0.01, $P < 0.005, #P < 0.001 |
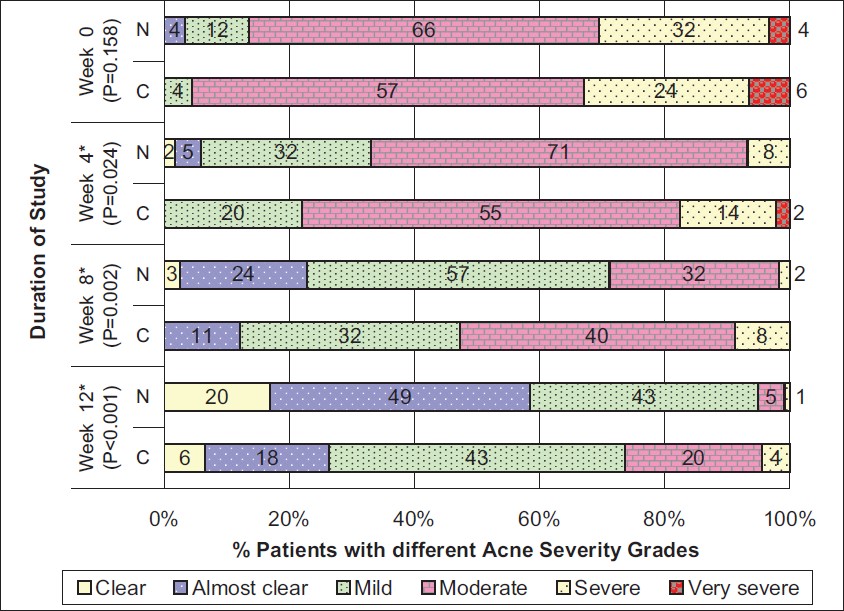 |
| Figure 3: The change in acne severity grades during the course of study in both the treatment groups, N = Nano-emulsion gel, C = Conventional gel, Group N: N = 118, Group C: N = 91, *Statistically significant P values |
 |
| Figure 4: The effect of adapalene 0.1% and clindamycin 1% nano-emulsion gel on acne lesions after 12 weeks of treatment (a and b at baseline, c at week 12) |

Safety analysis
No "serious" adverse event was reported in any of the patients enrolled in either of the study groups. None of the patients discontinued the study due to any adverse event in group N during the course of the study. On the other hand, 2 patients in group C (1 patient each after week 4 and week 8) discontinued the study due to adverse events of local irritation (i.e., stinging / burning sensation).
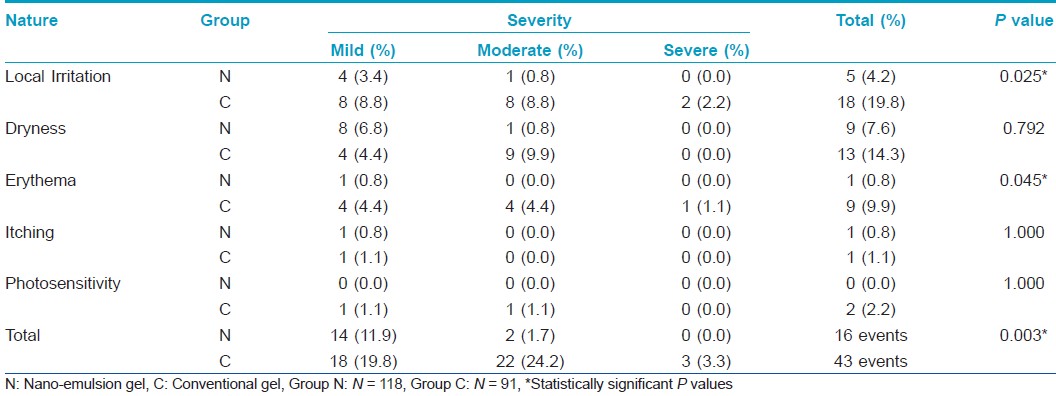
A total of 16 patients in group N and 33 patients in group C reported adverse events during the course of study. Thus, the patient adverse event rate was 13.6% [7.4% - 19.7%] in group N and 36.3% [26.4% - 46.1%] in group C, which was significantly different across the study groups (P < 0.001). The list of adverse events along with their severity reported by the patients in each of the treatment groups is shown in [Table - 4]. It was observed that significantly lesser number of patients in group N reported adverse events of local irritation and erythema as compared to those in group C (P = 0.025 and 0.045, respectively). Further, though there was no statistically significant difference in the number of patients reporting dryness in each of the study groups (P = 0.792), significantly lesser severity of dryness was reported in group N in comparison to group C (P = 0.011). 7 (43.8% [19.4% - 68.1%]) of 16 adverse events in group N and 32 (74.4% [61.4% - 87.5%]) of 43 events in group C were rated to have a "possible" association with the respective study medication while the remaining events were considered to have a "remote" association by the investigators (P = 0.027). All the adverse events settled completely with / without symptomatic treatment during the course of the study.
At the end of the study, 102 (86.4% [80.3% - 92.6%]) patients in group N and 58 (63.7% [53.9% - 73.6%]) patients in group C were rated to have an "Excellent" tolerability with the therapy (P < 0.001) according to the 4-point global assessment of tolerability scale. The complete overall assessment of tolerability is shown in [Figure - 5].
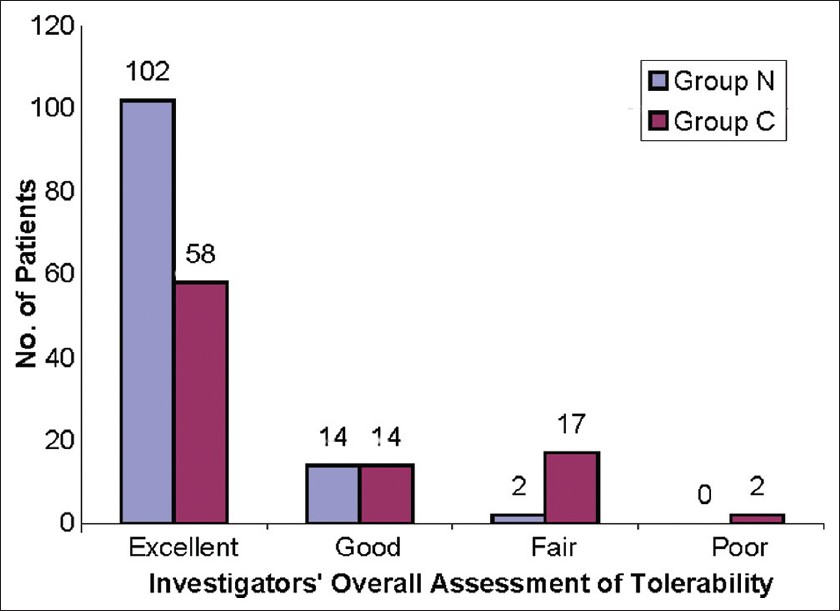 |
| Figure 5: The investigators' overall assessment of tolerability at the end of study, N = Nano-emulsion gel, C = Conventional gel, Group N: N = 118, Group C: N = 91, P<0.001 |
Discussion
The present study was carried out to assess the efficacy and safety of a novel nano-emulsion gel formulation of adapalene and clindamycin combination in comparison with its conventional formulation in Indian patients suffering from acne vulgaris of the face. The study has demonstrated that the novel formulation is more effective in reducing both the inflammatory as well as the noninflammatory acne lesions than the conventional one. Adverse events of local irritation reported with the novel formulation are also less frequent and milder in intensity than the comparator. Thus, the results indicate that adapalene and clindamycin combination nano-emulsion gel formulation produces better therapeutic response in the treatment of acne vulgaris of the face and also has a better safety profile than the conventional formulation.
Nano-emulsions have stable thermodynamic properties and do not have the problems of creaming, flocculation, coalescence or sedimentation, which are commonly associated with macro-emulsions, thus ensuring better stability and longer shelf-life of the formulation. [18] Nano-emulsions are one of the most promising drug transport systems since they increase the surface area of the drugs and thereby enhance their solubility as well as permeation. [19],[20] Recent in vitro and clinical studies have shown that nano-emulsions can improve penetration of active ingredients into the epidermis and dermis and exert direct bactericidal effects on P. acnes.[21],[22] They can permeate into pilo-sebaceous glands through hair follicles as well as into closed or infected comedones through lateral diffusion. [23] A synergistic action with topical antimicrobial agents, which can be helpful in the prevention of development of resistance, is also demonstrated. [24]
Further, while topical antimicrobials are generally well tolerated, local irritation with erythema, peeling and dryness are limiting factors in the use of topical retinoids. Adapalene is shown to be generally better tolerated than other topical retinoids and their improvised formulations, [7],[8],[9] and hydrating properties of the nano-emulsion formulation can further enhance the tolerability and acceptability of the topical preparation. [25] Treatment adherence has an important role in the success of acne therapy, and better tolerability can improve patients′ compliance to therapy. The present study endeavored to characterize the clinical benefits of the nano-emulsion gel formulation of adapalene and clindamycin combination.
This clinical trial had an open label design and therefore, can be influenced by the investigator bias; a disadvantage that is inherent to all open label studies. However, it is to be noted that proper blinding is difficult to achieve in active controlled studies with topical medications due to obvious differences in the preparations. Further, true blinding can only be achieved with complex study designs like double dummy technique, which has the disadvantage of twice-daily application and can adversely affect patient compliance. [26] In consideration of the above facts, an open label design was chosen for our active controlled study.
It was observed that the patient population receiving treatment with the conventional gel was younger than the one receiving the nano-emulsion gel in spite of implementation of random treatment allocation. Although baseline acne severity was comparable in both the study groups, younger age reportedly has some influence on the compliance to treatment. [26] However, compliance was ensured in both the study groups by out of sight examination of the medication containers and oral interview with the patients regarding the same. Moreover, retinoid-based combination therapy with antimicrobial agents is known to be effective and is uniformly recommended in all the age groups of patients. [7],[27]
A faster therapeutic response with significantly higher reductions in mean percentages of all acne lesions (total, inflammatory and noninflammatory) as well as in acne severity grades was noticeable as early as 4 weeks after treatment with nano-emulsion gel. Apart from the achievement of more than double treatment success rate at the end of the study, only 1 patient (0.8%) in group N and 4 patients (4.4%) in group C had grade 4 (severe) lesions at the end of week 12. Further, grade 3 (moderate) lesions were also present in fewer patients treated with the nano-emulsion gel (4.2% vs. 22.0%) at the end of the study due to significant reductions in the inflammatory acne lesions in their acne severity grades. Patients enrolled in the study had a greater number of noninflammatory lesions than inflammatory lesions which also reduced significantly more with the nano-emulsion gel. It was also noticeable that the treatment response did not reach a plateau in any of the treatment groups, and further treatment benefit can be expected with continued treatment of the combination therapy.
Nano-emulsion gel formulation of adapalene and clindamycin combination was well tolerated by the patients with a significantly lesser number and severity of adverse events reported as compared to the conventional formulation. Further, ′drug related′ adverse events were reported in a significantly lesser number of patients, and investigators′ overall assessment of tolerability also indicated a better safety profile of the novel formulation.
Earlier, Wolf et al.[14] and Zhang et al.[15] have studied topical combination therapy with adapalene 0.1% gel and clindamycin 1% lotion. Somewhat variable treatment response after 12 weeks of therapy is reported in these studies with % reductions in total, inflammatory and noninflammatory lesions, ranging from 46.7% - 75.1%, 55.0% - 75.2% and 42.5% - 75.5%, respectively. While treatment responses with conventional combination formulation have been within these ranges (62.7%, 71.4% and 58.4%, respectively), better reductions, particularly in total (79.7%) and inflammatory lesions (88.7%) are reported with the nano-emulsion gel in our study. Thus, a significant increase in efficacy is noteworthy with this novel nano-emulsion gel formulation for retinoid-based combination therapy.
On the tolerability aspect, Wolf et al. study reported that ~25% patients reported erythema and ~5% patients reported stinging / burning sensation of moderate to severe intensity with adapalene and clindamycin combination therapy. [14] In our study, a very low incidence and severity of erythema was reported, and 5.5% patients reported moderate to severe erythema with the conventional formulation only. These results may also reflect difficulty in perception of erythema in the Indian patients due to their generally darker complexion than the Caucasian patient population, apart from the difference in tolerability of the study medications. Further, moderate to severe local irritation was reported in only 0.8% patients with the nano-emulsion gel formulation, which is suggestive of an improvement in the tolerability profile.
Thus, the results of the present study suggest that the nano-emulsion gel formulation leads to a faster and significantly better response on the inflammatory lesions present in the higher severity grades of acne. An improved penetration of clindamycin into the infected pilo-sebaceous units with synergistic effects of nano-emulsion itself could be responsible for these findings. Noninflammatory lesions also responded well to the adapalene component of the combination. Moisturizing effects of the nano-emulsion gel formulation along with enhanced anti-inflammatory properties of adapalene as well as clindamycin in the pilo-sebaceous glands can be responsible for the improved local tolerance of the preparation as compared to the conventional gel formulation. Thus, this formulation appears to be a promising development in the treatment of acne vulgaris with an improved efficacy and tolerability profile.
Limitations of our study include open label design, which has an inherent possibility of investigator bias. Further, lack of matching for age between the 2 study groups may also have confounding effect on treatment response due to unexplained factors other than treatment compliance. Moreover, our study enrolled patients suffering from mixed type of acne lesions; therefore, treatment response in patients with predominantly noninflammatory or inflammatory acne can be elaborated with future studies in these specific subsets of patients. Further, studies with more vigorous study designs such as double blind, double dummy technique or split-face comparisons, extended for study duration longer than 12 weeks, are required to further elucidate the comparative treatment benefits of this novel nano-emulsion gel formulation.
In conclusion, the results of this randomized, open label, active-controlled, multicentric, phase IV clinical trial suggest that a nano-emulsion gel formulation of adapalene 0.1% and clindamycin 1% combination is more efficacious and better tolerated than its conventional formulation for the treatment of acne vulgaris in Indian patients. Further studies are warranted to confirm the therapeutic benefits of this nano-emulsion formulation.
Acknowledgements
Anil J. Jaiswal, Prafulla R. Pawar, Jay Kothari, S. Sreekanth, Reena Ved, Dhruv Patel, all from Cadila Healthcare Ltd. The authors would like to thank the patient for providing consent to use his photographs in this article.
| 1. |
Magin P, Adams J, Heading G, Pond D, Smith W. Psychological sequelae of acne vulgaris: Results of a qualitative study. Can Fam Physician 2006;52:978-9.
[Google Scholar]
|
| 2. |
Kokandi A. Evaluation of acne quality of life and clinical severity in acne female adults. Dermatol Res Pract 2010;2010:pii: 410809. [3 pages]. Article ID: 410809. doi:10.1155/2010/410809. Cited in PubMed; PMID 20706683. Available at: http://www.hindawi.com/journals/drp. Accessed June 6, 2011.
[Google Scholar]
|
| 3. |
Dunn LK, O'Neill JL, Feldman SR. Acne in adolescents: Quality of life, self-esteem, mood, and psychological disorders. Dermatol Online J 2011;17:1. [about 10 pages] Article ID: 1. Cited in PubMed; PMID 21272492. Available at: http://dermatology.cdlib.org. Accessed June 6, 2011.
[Google Scholar]
|
| 4. |
Al Robaee AA. Assessment of general health and quality of life in patients with acne using a validated generic questionnaire. Acta Dermatovenerol Alp Panonica Adriat 2009;18:157-64.
[Google Scholar]
|
| 5. |
Adityan B, Thappa DM. Profile of acne vulgaris-a hospital-based study from South India. Indian J Dermatol Venereol Leprol 2009;75:272-8.
[Google Scholar]
|
| 6. |
Friedlander SF, Eichenfield LF, Fowler JF Jr, Fried RG, Levy ML, Webster GF. Acne epidemiology and pathophysiology. Semin Cutan Med Surg 2010;29:2-4.
[Google Scholar]
|
| 7. |
Thiboutot D, Gollnick H, Bettoli V, Dréno B, Kang S, Leyden JJ, et al. New insights into the management of acne: An update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol 2009;60:S1-50.
[Google Scholar]
|
| 8. |
Gollnick H, Cunliffe W, Berson D, Dreno B, Finlay A, Leyden JJ, et al. Management of acne: A report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol 2003;49:S1-37.
[Google Scholar]
|
| 9. |
Thielitz A, Abdel-Naser MB, Fluhr JW, Zouboulis CC, Gollnick H. Topical retinoids in acne-an evidence-based overview. J Dtsch Dermatol Ges 2008;6:1023-31.
[Google Scholar]
|
| 10. |
Irby CE, Yentzer BA, Feldman SR. A review of adapalene in the treatment of acne vulgaris. J Adolesc Health 2008;43:421-4.
[Google Scholar]
|
| 11. |
Del Rosso JQ, Schmidt NF. A review of the anti-inflammatory properties of clindamycin in the treatment of acne vulgaris. Cutis 2010;85:15-24.
[Google Scholar]
|
| 12. |
Simonart T, Dramaix M. Treatment of acne with topical antibiotics: lessons from clinical studies. Br J Dermatol 2005;153:395-403.
[Google Scholar]
|
| 13. |
Jain GK, Ahmed FJ. Adapalene pretreatment increases follicular penetration of clindamycin: In vitro and in vivo studies. Indian J Dermatol Venereol Leprol 2007;73:326-9.
[Google Scholar]
|
| 14. |
Wolf JE Jr, Kaplan D, Kraus SJ, Loven KH, Rist T, Swinyer LJ, et al. Efficacy and tolerability of combined topical treatment of acne vulgaris with adapalene and clindamycin: a multicenter, randomized, investigator-blinded study. J Am Acad Dermatol 2003;49:S211-7.
[Google Scholar]
|
| 15. |
Zhang JZ, Li LF, Tu YT, Zheng J. A successful maintenance approach in inflammatory acne with adapalene gel 0.1% after an initial treatment in combination with clindamycin topical solution 1% or after monotherapy with clindamycin topical solution 1%. J Dermatolog Treat 2004;15:372-8.
[Google Scholar]
|
| 16. |
Leyden JJ, Krochmal L, Yaroshinsky A. Two randomized, double-blind, controlled trials of 2219 subjects to compare the combination clindamycin/tretinoin hydrogel with each agent alone and vehicle for the treatment of acne vulgaris. J Am Acad Dermatol 2006;54:73-81.
[Google Scholar]
|
| 17. |
Thiboutot D, Zaenglein A, Weiss J, Webster G, Calvarese B, Chen D. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol 2008;59:792-800.
[Google Scholar]
|
| 18. |
Shafiq S, Shakeel F, Talegaonkar S, Ahmad FJ, Khar RK, Ali M. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur J Pharm Biopharm 2007;66: 227-43.
[Google Scholar]
|
| 19. |
Shakeel F, Baboota S, Ahuja A, Ali J, Shafiq S. Skin permeation mechanism and bioavailability enhancement of celecoxib from transdermally applied nanoemulsion. J Nanobiotechnology 2008;6:8. [11 pages]. Article ID: 8. doi:10.1186/1477-3155-6-8. Cited in PubMed; PMID 18613981. Available at: http://www.jnanobiotechnology.com. Accessed June 6, 2011.
[Google Scholar]
|
| 20. |
Shakeel F, Baboota S, Ahuja A, Ali J, Aqil M, Shafiq S. Nanoemulsions as vehicles for transdermal delivery of aceclofenac. AAPS PharmSciTech 2007;8:E104. [9 pages] Article ID: 104. doi:10.1208/pt0804104. Cited in PubMed; PMID 18181525. Available at: www.aapspharmscitech.org. Accessed June 6, 2011.
[Google Scholar]
|
| 21. |
Leyden JJ, Ijzerman MM, Flack MR, Baker JR. Evaluation of the safety and efficacy of a novel anti-acne nanoemulsion (NB-003) in a randomized, open-label, parallel-group, single center study. Proceedings of the 50th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); 2010 Sep 12-15; Boston, U.S.A. Available at: http://www.nanobio.com/news/documents/ICAAC_2010_L1_1751_Acne.pdf. Accessed June 6, 2011.
[Google Scholar]
|
| 22. |
Ciotti S, Eisma R, Pannu J, McCarthy A, Baker JR. Novel Follicular-Targeted Nanoemulsions for Acne. Proceedings of Summer Academy Meeting 2009; 2009 Jul 29-Aug 2; Boston, U.S.A. Available at: http://www.nanobio.com/documents/AAD_Summer_P109_Follicular_Targeted_NE_Acne.pdf. Accessed June 6, 2011.
[Google Scholar]
|
| 23. |
Pannu J, Ciotti S, Eisma R, Ma L, Sutcliffe J. In vitro Susceptibility of Propionibacterium acnes and Skin Permeation of NB-00X Formulations. Proceedings of the 69th Annual Meeting of Society for Investigative Dermatology; 2009 May 6-9; Montreal, Canada. Available at: http://www.nanobio.com/news/documents/148098_616_11x17.pdf. Accessed June 6, 2011.
[Google Scholar]
|
| 24. |
Pannu J, McCarthy A, Martin A, Sutcliffe J. Susceptibility of Propionibacterium acnes in the Presence of Sebum to NB-003 Formulations. Proceedings of Summer Academy Meeting 2009; 2009 Jul 29-Aug 2; Boston, U.S.A. Available at: http://www.nanobio.com/documents/AAD_Summer_P106_Susceptibility_P_acnes_Sebum.pdf. Accessed June 6, 2011.
[Google Scholar]
|
| 25. |
Sonneville-Aubrun O, Simonnet JT, L'Alloret F. Nanoemulsions: A new vehicle for skincare products. Adv Colloid Interface Sci 2004;108-109:145-9.
[Google Scholar]
|
| 26. |
Zaghloul SS, Cunliffe WJ, Goodfield MJ. Objective assessment of compliance with treatments in acne. Br J Dermatol 2005;152:1015-21.
[Google Scholar]
|
| 27. |
Zaenglein AL, Thiboutot DM. Expert committee recommendations for acne management. Pediatrics 2006;118:1188-99.
[Google Scholar]
|
Fulltext Views
4,089
PDF downloads
1,467





