Translate this page into:
Eberconazole - Pharmacological and clinical review
2 Department of Dermatology, Fr Muller Medical College, Mangalore, India
Correspondence Address:
Latha Subramanya Moodahadu-Bangera
G-5, Mount Meru Apt Road, 5, Avenue 7, Banjara Hills, Hyderabad - 500 034
India
| How to cite this article: Moodahadu-Bangera LS, Martis J, Mittal R, Krishnankutty B, Kumar N, Bellary S, Varughese S, Rao PK. Eberconazole - Pharmacological and clinical review. Indian J Dermatol Venereol Leprol 2012;78:217-222 |
Introduction
Constant exposure to the ubiquitous fungi has made man vulnerable to fungal infections. Fungal infections of the skin are prevalent globally, with 20-25% of the world′s population being affected. [1] Life-time risk of acquiring a dermatophytic infection is estimated to be 10-20%. [2] Climatic conditions, cultural and socioeconomic factors not only contribute to the increased prevalence in tropics but also influence the type of fungal infection in a particular geographic area. [3]
Dermatophytosis is superficial skin infection caused by dermatophytes (specifically Trichophyton, Epidermophyton and Microsporum species), which attach to keratin, colonize the keratinized tissue using keratin as nutrition. [4] Since the infection is limited to the superficial layers of skin, topical application of antifungal agents is helpful in the treatment. Selecting a suitable antifungal agent is difficult as the market is flooded with many products. Moreover, treatment has become challenging and complex due to difficulties in diagnosis, deviation from classical clinical presentation, emergence of drug resistance, poor compliance and increase in immuno-suppressed conditions. With effective treatment, the cure rate is high, even though relapses and exacerbations are common.
Currently fungal infections are treated with either oral or topical antifungal agents depending upon the site and severity of infection. Oral antifungal agents are used only to treat widespread skin lesions, systemic fungal infections and in cases unresponsive to topical therapy.
Topical preparations with good local bioavailability are the most commonly used and preferred first line agents in the treatment of localized dermatomycosis. Their better efficacy which aims to shorten the treatment period, fewer side effects, minimizes recurrence, and ease of application enhances patient compliance resulting in better therapeutic response.
Eberconazole, a newer antimycotic agent, an imidazole derivative, initially designed and investigated in Spain by the Wassermann investigation centre, later acquired by Laboratories Salvat, was launched in 2005 for the treatment of cutaneous fungal infections. In this article we have reviewed the pharmacology and compared the clinical efficacy of eberconazole with other antifungal agents commonly used in clinical practice.
Eberconazole
Structure
Eberconazole ((1-(2,4-dichloro-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5-yl)-1H-imidazole)) [5] [Figure - 1], with a molecular formula C 18 -H 14 -C l2 -N 2 , is a broad-spectrum antifungal agent.
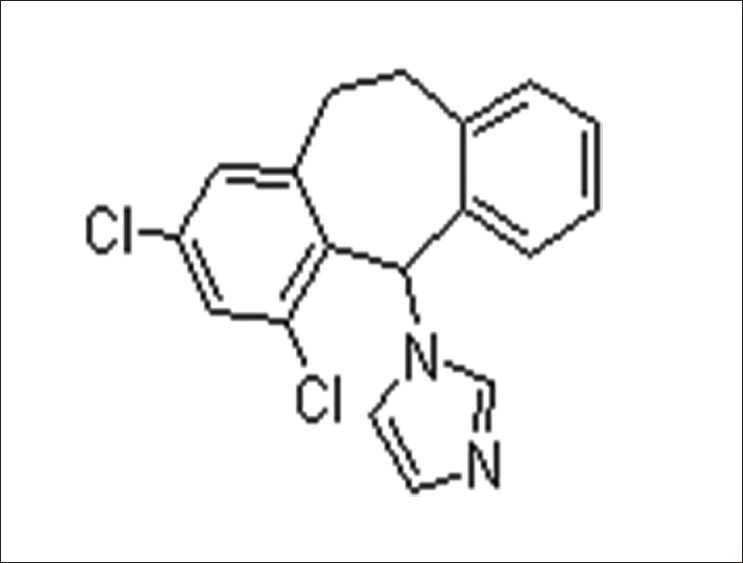 |
| Figure 1: Molecular structure of Eberconazole (Source: ChemBlink available from http://www.chemblink.com/products/128326-82-9.htm accessed on 10 October 2011) |
Spectrum of activity
Eberconazole has been shown to have broad antimicrobial spectrum of activity in vitro,[6] to be effective in dermatophytosis, candidiasis, infection by other yeasts such as Malassezzia furfur and causative agents of pityriasisversicolor in in vitro and animal studies. [6],[7],[8] Its effectiveness against most triazole-resistant yeasts (Candida krusei and Candida glabrata)[9] and also fluconazole resistant Candida albicans has been demonstrated in vitro.[7] It has also been shown to be effective against Gram-positive bacteria. [8]
Eberconazole is distinct from other imidazoles as it has been to shown to have anti-inflammatory activity, [7] which favors its use in the management of inflamed dermatophytic infections.
Mechanism of action
Eberconazole exerts fungicidal or fungistatic activity depending on concentration, being fungicidal at higher concentration and fungistatic at lower concentrations.
Eberconazole inhibits fungal growth by inhibiting ergosterol synthesis, an essential component of the fungal cytoplasmic membrane leading to structural and functional changes. It inhibits the fungal ergosterol synthesis by inhibiting lanosterol 14α-demethylase enzyme that is responsible for the formation of 14 α-methylsterols (precursor of ergosterols). Studies have shown that eberconazole binds to the phospholipid fraction of the cell and affects sterol synthesis intracellularly. [10] At high concentrations, it causes the leakage of small molecules such as potassium ions, amino acids, inorganic phosphate and nucleotides from the fungal cell leading to cell death.
The anti-inflammatory activity comparable to acetyl salicylic acid and ketoprofen, [7] shown in vivo by eberconazole is attributable to the inhibition of 5-lipooxygenase and to a lesser extent of cyclooxygenase-2. [10]
In vitro studies
Fernandez-Torres et al. compared the in vitro activity of eberconazole with that of clotrimazole, ketoconazole and miconazole against 200 strains of dermatophytes belonging to 19 species of fungi. Among the four drugs tested, eberconazole exhibited the lowest MIC for majority of the dermatophytic strains (P<0.05), suggesting an advantage of eberconazole over other widely used agents. [11]
In another in vitro study, eberconazole was shown to be as efficacious as or even better than clotrimazole and ketoconazole against various strains of Candida especially C. krusei and C. glabrata, which are usually resistant to triazoles. [7]
Preclinical studies
In preclinical studies using experimental models of superficial fungal infections, eberconazole has been shown to exhibit similar efficacy to clotrimazole, ketoconazole and better efficacy than bifonazole. [10] The studies showed that eberconazole is well tolerated without any delayed hypersensitivity or photosensitivity reactions. Also, no phototoxic effects were seen and no significant systemic absorption was observed with eberconazole. [7],[12]
Human pharmacokinetics
After topical application of eberconazole 2% cream in healthy volunteers, its concentration in human plasma and urine were below the lower limits of detection (<1.1 ng/ml for plasma and <1.0 ng/ml for urine) when analyzed by high performance liquid chromatography. [10]
However, data on metabolism and excretion of topical eberconazole are not available. [7]
Efficacy
Efficacy and safety of topical eberconazole have been established by a number of studies. Twice daily application of eberconazole 1% was found to be as efficacious as eberconazole 2% in 60 patients with mycologically proven tinea corporis and tinea cruris, in a phase II pilot study, but the measured parameters did not show any statistical significance. Overall clinical cure rate post therapy was 73.3-93.3% in different groups. Adverse effects were seen more with eberconazole 2% than with 1%, but without any statistical significance. [13]
Further, efficacy of eberconazole has been compared with the widely used topical antifungals in various studies. In a double blind study, eberconazole 1% cream′s therapeutic efficacy and safety profile was similar to miconazole. After 4 weeks of therapy, 76.09% patients on eberconazole showed effective response compared to 75.0% in miconazole group [Table - 1]. [10] Therapeutic effects of eberconazole with clotrimazole and miconazole are compared in [Table - 2]. [14] In another multicentric double blind randomized study, Repiso Montero et al. compared the the efficacy of eberconazole 1% cream with miconazole 2% cream in the treatment of dermatophytoses. In this study, eberconazole showed similar efficacy and safety profile with miconazole in the treatment of dermatophyte infection. This study also suggested that eberconazole can be considered a good alternative for the treatment of dermatophytoses as it has good safety and tolerability profile. [15]


Another double blind, randomized control study showed that the overall efficacy and safety of eberconazole cream in patients with cutaneous mycoses including dermatophytosis, candidiasis and pityriasis versicolor, was slightly higher (PP: 74.3% ITT: 61.7%) in comparison to clotrimazole (PP: 67.8% ITT:58.4%) at week 10, though the differences were statistically not significant. However, eberconazole showed a significantly greater (P=0.011) efficacy than clotrimazole in the treatment of dermatophytosis. Of 94.5% culture-positive patients (eberconazole 95.4%, clotrimazole 93.8%) at baseline, only 25.9% at week 4 and 23.9% at week 10 remained culture positive (eberconazole 21.5%, clotrimazole 24.3%). There was no statistically significant difference between the rate of reinfection and relapse in both the groups. [9] Subgroup analysis of the same study showed that in ITT population, clinical efficacy (defined as the percentage effective response at week 4 of treatment) was higher with eberconazole (66.17%) than with clotrimazole (60.63%). In patients with pityriasis versicolor and candidiasis, no significant difference in therapeutic efficacy was noted with both agents [Table - 3]. [10],[16] Secondary analysis which included symptomatology, time to cure, results at week 4 and the investigators impression, did not show any statistically significant differences. [10] In another study, eberconazole was more effective against dermatophytosis with a response rate of 61% (for clotrimazole 46%) than candidiasis (effective response for clotrimazole 73% and eberconazole 50%). Overall efficacy was 72% for eberconazole and 61% for clotrimazole. There was no significant difference in relapse in both groups, 4% in clotrimazole and 1% relapse in eberconazole was noted. [17]

A phase III Indian study (n=92) with a treatment duration of 4 weeks followed by another 4 weeks follow-up, confirmed the earlier findings that eberconazole 1% is significantly effective against cutaneous dermatomycoses. The therapeutic efficacy was 97.44%; cure rate defined as negative microscopy and culture and reduction in total symptom score ≤1 was 84.62% and improvement defined as negative microscopy and culture and reduction in total symptom score=2 was 12.82%. Only one patient failed to respond to treatment (failure to respond was defined as negative microscopy and culture and reduction in total symptom score > 2 or microscopic and/or culture positive, independent of total symptom score). Clinical improvement was seen within 2 weeks of therapy indicating an early response with eberconazole. Its good safety profile was confirmed as only one patient reported mild burning sensation over the site of application, which disappeared with continued treatment (Data on file). [18]
Safety
In a phase I study of single and multiple doses of topical eberconazole 2% use, no significant and evaluable changes were noted in vital signs, blood or urine biochemical parameters of the volunteers. Eberconazole was not detected either in plasma or urine indicating no significant systemic absorption. [10]
Another phase I study demonstrated that topical eberconazole 1% (0.1 g), does not induce photosensitivity and phototoxicity indicating its good tolerability. [19]
On topical application, safety, tolerability and adverse event (AE) profile of eberconazole was similar to that of placebo. On single dose application, mild symptoms which were common to eberconazole and placebo were reported within the first hour. On multiple dose application, mild pruritus, occasional burning sensation, and mild skin dryness were seen with eberconazole and placebo as well, without showing a dose effect relationship. In the objective evaluation, the only change observed was slight erythema that appeared for the first time on day 3 (lasting 3days). Later on day 9, two volunteers reported reddening (zone covered with eberconazole 2%) with light granulation associated with a mild pruritus and burning sensation of 1 and 3 days duration, and on day 10 one volunteer presented with light erythema that lasted 12 hours. [10]
Further, in a phase II study, eberconazole shared similar safety profile with clotrimazole, as there was no significant difference in the occurrence of AEs. Erythema and pruritus were the commonly seen AEs in this study [Table - 4]. Mild irritation was seen in three of 60 patients and two patients withdrew from the study due to severe skin irritation. [13] In a phase III study, 20% patients in eberconazole group and 26% in clotrimazole group withdrew prematurely due to AEs in patients with candidiasis. [17] In another phase III study, local cutaneous irritation was seen in 6.1% of patients in eberconazole group and in 3.7% in clotrimazole group without any systemic or serious events and the difference between the two groups was not significant. [10]

In two studies which evaluated eberconazole for its a) topical and general tolerability, b) eventual development of sensitization, c) local availability, and d) degree of systemic absorption, the only change observed was slight redness in a few volunteers after both active and placebo applications. This remitted spontaneously without intervention and the study was continued with the administration of repeated increasing doses. A few participants described mild side effects which occurred in areas where placebo or eberconazole were applied, mainly within the first hour of application. The most frequent adverse effect after the first application was coldness, and after repeated increasing-doses was itching. No signs or symptoms of skin reactivity were observed following re-exposure to the product. Vital signs (systolic and diastolic blood pressure, heart rate and body temperature), ECG, or analytical parameters (clinical hematology and biochemistry) did not show any clinically significant or relevant changes. The quantity of compound collected through washing gauzes decreased progressively over time. Plasma and urine concentrations of eberconazole were below the quantification limits of the analytical method at all times. This study has confirmed that eberconazole cream is a topical anti-mycotic drug having good local and general tolerability. It also showed acceptable topical availability without detectable systemic drug levels, and did not appear to cause skin sensitivity. [20] No phototoxicity or photosensitivities were seen with topical application of eberconazole1% cream. [7]
Formulation advantage
Eberconazole has been marketed as a cream with a characteristic lipophilic-hydrophilic molecular structure for better penetration of fungal cell membrane and prolonged duration of action. The galenic components of this topical azole favor and optimize the drug′s action in the skin, fatty acid esters facilitate penetration in the skin and make the cream easy to spread, while polyacrylamides produce a filmogenous effect and facilitate the continuance of the active principle in the skin. [12]
Discussion
Eberconazole′s broad spectrum of anti-mycotic activity against yeast and fungi, high efficacy in the preclinical studies led to its clinical development to explore safety and efficacy in humans. Its anti-inflammatory action and efficacy against gram positive bacteria can add to its efficacy in inflamed cutaneous mycoses and in secondary infections, favoring the regression of inflammatory symptoms and treatment compliance.
Eberconazole is clinically effective in the treatment of topical fungal infections, with a good safety profile and good tolerability. It has acceptable topical availability with no detectable systemic drug levels, and does not appear to cause skin sensitivity. [20]
Eberconazole was more active in vitro against a broad range of dermatophyte species than the other topical drugs tested suggesting that it may be a good alternative for the topical treatment of dermatophytoses. [11] Various clinical studies have documented its efficacy in the treatment of dermatophytosis. Eberconazole showed greater therapeutic efficacy than clotrimazole 1% and equivalent efficacy with miconozole 2% in the management of dermatophytosis and was similar in efficacy in the management of cutaneous candidiasis and pityriasis versicolor. [12]
The results of study in Indian patients [17] has confirmed the earlier findings that eberconazole 1% is significantly effective against cutaneous dermatomycoses and the reported AEs were similar to that seen in other studies indicating that it is well tolerated in majority of patients.
Data, available on eberconazole, is quite limited. Data on its metabolism and excretion are still not available [7] thereby requiring further studies. Although it is effective against triazole resistant yeasts, [8] it is found to be less effective clinically compared to clotrimazole 1% [16] thereby limiting its use in this condition. Moreover, comparative efficacy is yet to be established with allylamines and other new antifungal agents.
The rise in fungal infections due to increase in incidence of immunocompromised states, change in socioeconomic and cultural states is demanding an effective antimycotic agent for the treatment and cure. Superficial fungal infections being more prevalent in tropical countries, an antimycotic agent having good safety profile and better efficacy with less chance of developing drug resistance such as eberconazole is always welcome.
With newer antifungal agents flooding the market, eberconazole′s effectiveness in the treatment of a wide range of cutaneous fungal infection in comparison with the existing and newer agents has to be established to determine its place in the topical therapy of fungal infections in future.
Conclusions
Complexities in the treatment of dermatomycosis have compelled the invention of newer antifungal agents with better efficacy and safety profile. Proven better efficacy, good safety and tolerability profile of eberconazole along with lack of sensitizing ability make it an attractive and suitable alternative in the management of dermatomycosis. However, further comparative studies with other commonly used agents are required to position eberconazole among topical antifungals.
| 1. |
Havlickova B, Czaika VA, Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses 2008;51:2-15.
[Google Scholar]
|
| 2. |
Drake LA, Dinehart SM, Farmer ER, Goltz RW, Graham GF, Hardinsky MK, et al. Guidelines of care for superficial mycotic infections of the skin: tinea corporis, tinea cruris, tinea faciei, tinea manuum, and tinea pedis. J Am Acad Dermatol 1996;34:282-6.
[Google Scholar]
|
| 3. |
Charles AJ. Superficial cutaneous fungal infections in tropical countries. Dermatol Ther 2009;22:550-9.
[Google Scholar]
|
| 4. |
Nelson MM, Martin AG, Heffernan MP. Superfecial fungal infections: Dermatophytosis, Onychomycosis, Tinea Nigra, Piedra. In: Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, editors.Fitzpatrick's Dermatology in General Medicine. 6 th ed. New York: McGraw Hill; 2003. p. 1989.
th ed. New York: McGraw Hill; 2003. p. 1989.'>[Google Scholar]
|
| 5. |
Eberconazole [internet]. North Carolina: ChemBlink Inc.; Available from: http://www.chemblink.com/products/128326-82-9.htm. [last cited on 2011 Oct 10].
[Google Scholar]
|
| 6. |
Zalacain A, Obrador C, Martinez JP, Vinas M, Vinuesa T. Characterization of the antimicrobial susceptibility of fungi responsible for onychomycosis in Spain. Med Mycol 2011;49:495-9.
[Google Scholar]
|
| 7. |
Thomson Reuters Pharma™ [Internet]. Drug report: Eberconazole. Thomson Reuters Pharma™. Updated on 12/Aug/2010.
[Google Scholar]
|
| 8. |
Font E, Freixes J, Julve J. Profile of a new topical antimycotic, eberconazole. Revista iberoamericana De Micologia 1995;12:16-7.
[Google Scholar]
|
| 9. |
Rubin AI, Bagheri B, Scher RK. Six novel antimycotics. Am J Clin Dermatol 2002;3:71-81.
[Google Scholar]
|
| 10. |
Laboratorios Salvat, S.A (Innovators). Product information. 2001.
[Google Scholar]
|
| 11. |
Fernández-Torres B, Inza I, Guarro J. In vitro activities of the new antifungal drug eberconazole and three other topical agents against 200 strains of dermatophytes. J Clin Microbiol 2003;41:5209-11.
[Google Scholar]
|
| 12. |
Martin-Gonzalez B, Vilata-Corell JJ, Linares ML, Laguna-Argente C, Roche-Gamon E. Treatment in superficial cutaneous mycoses with eberconazole [Spanish]. Med Clin 2006;126:47-50.
[Google Scholar]
|
| 13. |
del Palacio A, Cuétara S, Rodríguez Noriega A. Topical treatment of tineacorporis and tineacruris with eberconazole (WAS 2160) cream 1% and 2%: a phase II dose-finding pilot study. Mycoses 1995;38:317-24
[Google Scholar]
|
| 14. |
High WA, Fitzpatrick JE. Topical antifungal agents. In: Freedberg IM, Eisen AZ, Wolff K, Austen KF, editors. Fitzpatrick's Dermatology in General Medicine. 7 th ed. New York: McGraw Hill; 2008. p. 2116-8.
th ed. New York: McGraw Hill; 2008. p. 2116-8.'>[Google Scholar]
|
| 15. |
Repiso Montero T, López S, Rodríguez C, del Rio R, Badell A, Gratacós MR. Eberconazole 1% cream is an effective and safe alternative for dermatophytosis treatment: multicenter, randomized, double-blind, comparative trial with miconazole 2% cream. Int J Dermatol 2006;45:600-4.
[Google Scholar]
|
| 16. |
Fonseca Capdevila E. Effectiveness of Eberconazole 1% cream as opposed to Clotrimazole 1% cream in patients with cutaneous mycosis. Skin 2004;19:480-4.
[Google Scholar]
|
| 17. |
del Palacio A, Ortiz FJ, Pérez A, Pazos C, Garau M, Font E. A double blind randomized comparative trial: eberconazole 1% cream versus clotrimazole 1% cream twice daily in Candida and dermatophyte skin infections. Mycoses 2001;44:173-80.
[Google Scholar]
|
| 18. |
Dr. Reddy's Laboratories Ltd. Efficacy and safety evaluation of Eberconazole in cutaneous dermatomycoses. CT report 2006.
[Google Scholar]
|
| 19. |
Rius-Tarruella J, López-Bertran R, Viayna-Cardona C. A phase I photosensitivity/phototoxicity study of eberconazole cream in twelve healthy subjects. Publicadona Piel 2005;20:314-6.
[Google Scholar]
|
| 20. |
Barbanoj MJ, Antonijoan R, Garcia-Gea C, Puntes M, Gich I, Jane F. Eberconazole cream: topical and general tolerability, sensitisation potential, and systemic availability. Met Find Exp Clin Pharmacol 2005;27:227-34.
[Google Scholar]
|





