Translate this page into:
Nail dermoscopy (onychoscopy) findings in the diagnosis of primary onychomycosis: A cross-sectional study
2 Department of Microbiology, University College of Medical Sciences and GTB Hospital (University of Delhi), Delhi, India
3 Department of Pathology, University College of Medical Sciences and GTB Hospital (University of Delhi), Delhi, India
Correspondence Address:
Archana Singal
Department of Dermatology and STD, University College of Medical Sciences and GTB Hospital (University of Delhi), Delhi - 110 095
India
| How to cite this article: Kayarkatte MN, Singal A, Pandhi D, Das S, Sharma S. Nail dermoscopy (onychoscopy) findings in the diagnosis of primary onychomycosis: A cross-sectional study. Indian J Dermatol Venereol Leprol 2020;86:341-349 |
Abstract
Background: Diagnosis of onychomycosis involves direct microscopic examination with potassium hydroxide, culture or histopathology with periodic acid–Schiff staining. Nail dermoscopy (onychoscopy) is a noninvasive, rapid and easily available diagnostic tool though its utility in onychomycosis remains unexplored.
Objective: To describe the various onychoscopic patterns and compare its percentage positivity with that of standard potassium hydroxide examination, culture and histopathology in patients with a clinical diagnosis of onychomycosis.
Methods: The study recruited 100 patients with a presumptive clinical diagnosis of onychomycosis. A detailed history, physical examination including that of nails and clinical photography was followed by onychoscopy with DermLite DL3. The nail clippings were sent for direct microscopic examination with potassium hydroxide, mycological culture and histopathology with periodic acid–Schiff stain. The patient was said to have onychomycosis if at least one of the three tests was positive.
Results: Onychomycosis was confirmed by potassium hydroxide and/or culture and/or histopathology in 88 patients. Onychoscopic features were identified and their association with different clinical variants of onychomycosis was attempted. Percentage positivity for diagnosing onychomycosis in decreasing order was: direct microscopic examination with potassium hydroxide followed by spiked pattern, subungual hyperkeratosis, distal irregular termination on onychoscopy, histopathology, mycological culture and ruins aspect again observed on onychoscopy.
Limitations: Small sample size.
Conclusions: Many onychoscopic features are highly specific for different variants of onychomycosis so onychoscopy may serve as an important and quick adjunct to diagnose onychomycosis until other time-consuming investigations, such as culture and periodic acid–Schiff become available. Studies on a larger population will help arrive at a logistic conclusion.
Introduction
Onychomycosis caused by dermatophyte, nondermatophytic molds or yeasts,[1] has a prevalence of 5% worldwide[2] and 0.5–5% in India.[3] Though its diagnosis is mainly clinical, laboratory confirmation is essential, as prolonged systemic treatment is needed in the majority. In addition, in the absence of laboratory confirmation, it is difficult to exclude treatment failure when there is lack of therapeutic response.[4] Diagnostic modalities for onychomycosis include direct microscopic examination with potassium hydroxide immunofluorescent microscopy with calcofluor white, fungal culture, histology with periodic acid–Schiff staining and polymerase chain reaction.[5] Dermoscopy of nail or onychoscopy is an upcoming diagnostic tool with ever-expanding indications beyond pigmented nail disorders. It is a noninvasive, quick, reproducible and office-based procedure. Limited literature exists pertaining to onychoscopic features of onychomycosis. Piraccini et al. described distinctive dermatoscopic features exclusive to distal lateral subungual onychomycosis.[6] Jesús-Silva et al. described the presence of spiked pattern, longitudinal striae pattern, linear edge pattern and distal irregular termination pattern in onychomycosis.[7]
In this study, we identified onychoscopic features in different clinical types of onychomycosis and compared its diagnostic utility with that of direct microscopic examination with potassium hydroxide, mycological culture and periodic acid–Schiff stained nail clippings.
Methods
Study type
It is a cross-sectional observational study carried out in the University College of Medical Sciences and GTB hospital during 2015–2017 and was approved by the institutional ethics committee.
Study participants
A hundred adult consenting patients with clinical features suggestive of onychomycosis were recruited. Patients with nail pathology attributable to noninfective preexisting disease and who had received topical or systemic antifungal in the last 1 and 3 months, respectively, were excluded. In addition, pregnant and lactating women and those with severe systemic co-morbidities were not included.
A detailed history and physical examination were carried out in each subject and findings were recorded in a predesigned case record form.
Clinical examination
A thorough examination of all the fingers and toenails for the various signs of onychomycosis was undertaken after cleansing with alcohol. Clinical photographs of the involved nails were taken and stored in JPEG format.
Onychoscopy
A single representative nail was chosen from each subject for onychoscopy. Onychoscopy with a DermLite DL3 dermatoscope (3Gen, San Jan Capistrano, CA, USA) attached to Sony Cybershot camera (DSC-W800 5x magnification) was performed, giving a magnification of 10x. Polarizing and non polarizing mode was used as needed and isopropyl alcohol was used as the interface medium.
Sample collection for direct microscopic examination, culture and histopathology
All samples were collected from the most proximal involved area of the affected nail to minimize contamination. Depending on the clinical presentation nail clippings, nail plate scrapings, subungual debris and nail bed scrapings were collected.[8]
On direct microscopic examination in 20% potassium hydroxide, long-branched, filamentous hyphae with or without arthrospores were observed in dermatophytic infection, pseudohyphae in candidal onychomycosis and mycelia, arthrospores or yeast cells in nondermatophytic mold onychomycosis. A part of the nail clipping was sent in a sterile envelope for inoculation on Sabouraud's dextrose agar medium with and without cycloheximide (0.5g/l), at 25°C for optimal growth. In the case of growth, colony characteristics were studied on lactophenol cotton blue wet mount preparation. The culture was considered negative in the absence of growth after 4 weeks of incubation. The most proximal part of the affected nail plate was biopsied, fixed in 10% buffered formalin solution and sent for histopathology for hematoxylin and eosin and periodic acid–Schiff staining. Mycological etiology was confirmed when neutrophilic exudates and crusts were evident on histopathology and red-colored fungal elements were appreciated in periodic acid–Schiff stained nail clippings.
Analysis
The data were coded and entered into Microsoft Excel. It was then analyzed for percentages, rates, proportions and association, using a Chi-square test of significance, where P < 0.05 was considered significant.
Results
A patient who showed evidence of fungal elements either on with potassium hydroxide, culture or histopathology was designated as a case of onychomycosis. Eighty-eight patients qualified to be cases of onychomycosis. The remaining 12 served as control.
Onychoscopy
Onycholysis was the most frequently observed feature in 85 (96.6%), followed by a spiked pattern in 76 (86.4%) [Figure - 1]. Chromonychia of the nail plate [Figure - 2] and subungual hyperkeratosis were seen in 75 (85.2%) each, dryness and scaling of the adjacent skin in 73 (83%) and distal irregular termination [Figure - 3] in 72 (81.8%). We observed ruins aspect [Figure - 4] in 52 (59.1%) and longitudinal striae [Figure - 5] in 22 (25%) cases. The association of spiked pattern, subungual hyperkeratosis, distal irregular termination and ruins aspect with onychomycosis, was statistically significant (P < 0.05) [Table - 1].
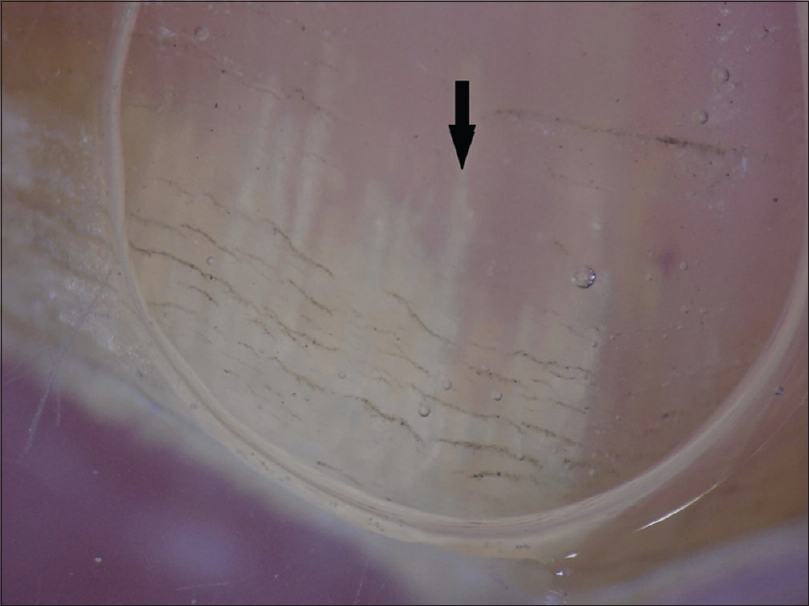 |
| Figure 1: Dermatoscopic image of spiked pattern (arrow): polarizing mode with the interface (magnification: 10x) |
 |
| Figure 2: Dermatoscopic image of chromonychia: polarizing mode with the interface (magnification: 10x) |
 |
| Figure 3: Dermatoscopic image of distal irregular termination (arrow) with onycholysis (magnification: 10x) |
 |
| Figure 4: Dermatoscopic image of subungual hyperkeratosis with ruins aspect (magnification: 10x) |
 |
| Figure 5: Dermatoscopic image of longitudinal striae pattern (arrow) (magnification: 10x) |

In our study, we came across the following new patterns on onychoscopy [Table - 2].

- Lamellar micro-splitting- It appeared as the lamellar splitting of the nail plate on onychoscopy but was not appreciable on naked-eye-examination. This feature as a surface finding was appreciated on nonpolarizing onychoscopy and was observed in 9/11 (81.8%) cases of total dystrophic onychomycosis (P = 0.05) [Figure - 6].
- Leukonychia- We classified the finding of leukonychia as:

Figure 6: Dermatoscopic image of lamellar micro-splitting (arrow) (magnification: 10x) - White fluffy shadows: Cloudiness/cotton-like white shadow, present at different levels of the nail plate and was better appreciated on polarizing mode [Figure - 7]. It was present in 9/11 (81.8%) of total dystrophic onychomycosis, 44/72 (61.1%) of distal lateral subungual onychomycosis, 2/2 (100%) proximal subungual onychomycosis and 1/3 (33.3%) case with superficial onychomycosis
- Transverse striate pattern- Horizontally placed white striate opacities seen on nonpolarizing onychoscopy in all clinical variants of onychomycosis [Figure - 8]
- Homogenous opacity- Uniform opacity involving a part of the nail plate or the entire surface of the nail plate was seen in all clinical variants [Figure - 9].
 |
| Figure 7: Dermatoscopic image of white fluffy shadow (arrow): polarizing mode with the interface (magnification: 10x) |
 |
| Figure 8: Dermatoscopic image of transverse striate pattern leukonychia: polarizing mode with the interface (magnification: 10x) |
 |
| Figure 9: Dermatoscopic image of homogenous opacity (polarizing mode with the interface) (magnification: 10x) |
As endonyx was not observed among our patients, associations with the same could not be established. We found no associations of the previously mentioned features with the causative organisms.
Onychoscopy and clinical variants
The frequency of onychoscopic pattern with each clinical variant was noted [Table - 3]. Following significant associations (P < 0.05) were noted with regard to the onychoscopic features-

- Distal lateral subungual onychomycosis- Onycholysis, spiked pattern and absence of homogenous opacity
- Total dystrophic onychomycosis- Ruins aspect and longitudinal striae
- Proximal subungual onychomycosis- No statistically significant association could be achieved for proximal subungual onychomycosis and the onychoscopic features
- Superficial onychomycosis- Presence of homogenous opacity, absence of spiked pattern (subungual hyperkeratosis), ruins aspect
- The association of lamellar micro-splitting and total dystrophic onychomycosis though not statistically significant (P = 0.05), was evident in 81.8% of total dystrophic onychomycosis.
Onychoscopic features in the control group
Among the 12 control who did not suffer from onychomycosis, onycholysis was seen in all cases with the spiked pattern being present in 5 (41.7%). Subungual hyperkeratosis was seen in seven (58.3%) cases with ruins aspect being present in only one case. The distal edge of the nail plate had a regular pattern i.e. distal regular pattern six (50%) cases. The longitudinal striae pattern was evident in two (16.7%). The association of distal regular pattern was significant for ruling out onychomycosis (P < 0.05).
Discussion
Onychoscopy is a simple, rapid, office-based diagnostic tool to diagnose onychomycosis as compared with time-consuming and labor-intensive methods like direct microscopic examination, culture or polymerase chain reaction. There are a limited number of studies of onychoscopy in distal lateral subungual onychomycosis variant of onychomycosis [Table - 4], whereas the present study included all clinical variants of onychomycosis.[6],[7],[9],[10],[11],[12]

Onycholysis was the most frequent observation present in 85 (96.6%) cases. The three cases where onycholysis was absent belonged to the superficial onychomycosis variant. Onychoscopy had the distinct advantage of delineating the pattern of the proximal edge of the onycholytic area. A spiked pattern was noted in 76/88 (86.4%), linear pattern of termination was seen in the rest 12/88 (13.6%). The association of spiked pattern with onychomycosis was found to be statistically significant (P < 0.05). This finding is concordant with previous studies conducted by Yadav and Khopkar (100%),[10] Piraccini et al. (100%),[6] Jesús-Silva et al. (23.8%)[7] and Nargis et al. (78.3%).[11] We observed chromonychia of the nail plate in 75/88 (85.2%) cases, most frequently being yellow (50%) followed by brown (27.3%), black (9.1%) and green (3.4%). In a recent study, similar pattern of chromonychia (yellow >brown >black: 70%>51%>24%) has been described.[6] They also described white chromonychia in 59% cases, a statistically significant finding in diagnosing onychomycosis. Previous studies have variously described white discoloration of the nail bed or plate as opacity (Di Crignis),[12] white chromonychia (Piraccini BM)[6]/leukonychia. In the present study, leukonychia has been studied separately in detail and classified into white fluffy shadows, transverse striate pattern or homogenous opacity. These morphological types of leukonychia, though present distinctly in cases with onychomycosis, have not been found to be statistically significant for diagnosing onychomycosis, probably because of smaller sample size. In addition, as onychoscopy of onychomycosis is in an evolutionary phase, a uniform nomenclature to describe patterns of chromonychia may be used in the future to arrive at conclusive evidence.
Subungual hyperkeratosis was observed in 75 (85.2%) cases, of which 52 (59.1%) showed ruins aspect/ruinous appearance. The association of subungual hyperkeratosis, as well as ruinous appearance with onychomycosis, was statistically significant (P < 0.05). Subungual hyperkeratosis with ruins aspect has been described in all 10 nails of onychomycosis studied by De Crignis et al.[12] and 7/21 (33.3%) nails studied by Yadav and Khopkar,[10] suggesting that subungual hyperkeratosis could be one of the key features with potential utility in diagnosing onychomycosis. Distal irregular termination of the nail plate was observed in 72 (81.8%) cases, with a significant association with onychomycosis (P < 0.05). This observation is in contrast with the study conducted in 2015 where 43.2% showed distal irregular termination.[7] This inconsistency in findings may be because of the occurrence of pulverization in severely affected nails and of long disease duration. Therefore, this finding needs to be explored with respect to the severity of the affected nail and its disease duration. We also noted dryness and scaling of the adjacent skin in 73 (83%) cases. It was unrelated to the presence of tinea pedis or tinea manuum. Infected nail and surrounding skin may be neglected about daily cleansing and grooming causing dryness and scaling. It may also be because of the onychopathy per se or may result from the application of topical medication. Though Nakamura and Costa studied dryness and scaling onychoscopy, it was not commented upon in detail.[9] In our study, irregularly placed fine transverse splitting of the nail plate was observed on onychoscopy in 45 (51.1%) of cases. This observation has not been described previously. We have named this finding as lamellar micro-splitting as it was not visualized on naked eye examination. Although it has not been found to be of any statistical significance in our study, detailed evaluation with a larger sample size could probably give results that are more conclusive.
Onychoscopic feature and clinical variant
In this study, we found a spiked pattern, longitudinal striae, linear onycholytic edge and distal irregular termination in distal lateral subungual onychomycosis in the frequency of 90.3%, 22.2%, 9.7% and 81.9%, respectively. Corresponding figures from a study by Jesús-Silva et al., conducted on 67 cases of distal lateral subungual onychomycosis were 25.3%, 62.6%, 19.4% and 38.8%, respectively.[7] We observed a significant association between distal lateral subungual onychomycosis and onycholysis, spiked pattern and absence of homogenous opacity.
We also appreciated spiked pattern, longitudinal striae, linear onycholytic edge and distal irregular termination in total dystrophic onychomycosis at a frequency of 90.9%, 54.5%, 9.1% and 100%, respectively. Jesús-Silva et al. observed that in 87 total dystrophic onychomycosis cases, 25.3% had a spiked pattern, 58.6% had longitudinal striae, 24.1% had linear edge and 47% had distal irregular termination.[7] In our study, we found a significant association of total dystrophic onychomycosis with ruins aspect and longitudinal striae. The dystrophy of the nail could ensue the ruinous appearance of subungual hyperkeratosis, which needs to be studied further. The longitudinal striae pattern is postulated to be because of the colors of fungal colonies, which may be better visible in a nail with higher infection and dystrophy. The association of total dystrophic onychomycosis with lamellar micro-splitting had a P = 0.05. This association needs further exploration with larger sample size.
Cases of superficial onychomycosis did not show onycholysis, spiked pattern, subungual hyperkeratosis or ruins aspect. The transverse striate pattern was present in all three nails of superficial onychomycosis. This feature of superficial onychomycosis should be evaluated at a larger scale. Absence of spiked pattern, subungual hyperkeratosis and ruins aspect were significantly associated with superficial onychomycosis.
The sensitivity and specificity of significant onychoscopic features [Table - 5] to diagnose onychomycosis were calculated with a sample size of 88/100 cases of onychomycosis, the remaining 12 serving as the negative control. The sensitivity for a spiked pattern for diagnosing onychomycosis was 86.4% and specificity was 58.3%. Corresponding figures for subungual hyperkeratosis were 85.2% and 41.7%, for distal irregular termination were 81.8% and 50% and for ruins aspect were 59.1% and 91.7%, respectively. The specificity of combined features [Table - 6] of spiked pattern and ruins aspect, ruins aspect and distal irregular termination was enhanced to 91.7% for each combination. Such a result makes way for larger studies wherein criteria can be established for diagnosing onychomycosis based on multiple onychoscopic features in combination.


The percentage positivity of direct microscopic examination with potassium hydroxide, mycological culture and histopathology were compared with previous studies [Table - 7]. Further, we compared percentage positivity of onychoscopic findings with that of direct microscopic examination with potassium hydroxide, mycological culture and histopathology with periodic acid–Schiff.[13],[14],[15],[16],[17],[18],[19],[20] We were unable to find a study drawing a comparison of these parameters. Taking only statistically significant onychoscopic findings into consideration, percentage positivity for diagnosing onychomycosis in the decreasing order was found as direct microscopic examination with potassium hydroxide 80(88.6%), spiked pattern 76 (88.4%), subungual hyperkeratosis 75 (85.2%) and distal irregular termination on onychoscopy 72 (81.8%), histopathology with periodic acid–Schiff 68 (77.2%), ruins aspect 52 (59.1%) and mycological culture 47 (53.40%).

Our study had a limitation of a smaller sample size, especially the number of controls, which decreased its statistical relevance.
Conclusions
Onychoscopic features like spiked pattern, distal irregular termination and ruins appearance have a significant association with onychomycosis and its variants. We identified would few newer features, like white fluffy shadows, transverse striate pattern, homogenous opacity and lamellar micro-splitting in the diagnosed cases of onychomycosis. More studies can be conducted with a larger sample size, to establish these associations with certainty.
Declaration of patient consent
The authors certify that they have obtained appropriate consent forms from all patients. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Kaur R, Kashyap B, Bhalla P. Onychomycosis-epidemiology, diagnosis and management. Indian J Med Microbiol 2008;26:108-16.
[Google Scholar]
|
| 2. |
Murray SC, Dawber RP. Onychomycosis of toenails: Orthopaedic and podiatric considerations. Australas J Dermatol 2002;43:105-12.
[Google Scholar]
|
| 3. |
Sobbanadri C, Rao DT, Babu KS. Clinical and mycological study of superficial fungal infections at government general hospital, Guntur and their response to treatment with hamycin, dermostatin and dermamycin. Indian J Dermatol Venereol 1970;36:209-14.
[Google Scholar]
|
| 4. |
Seebacher C, Brasch J, Abeck D, Cornely O, Effendy I, Ginter-Hanselmayer G, et al. Onychomycosis. Mycoses 2007;50:321-7.
[Google Scholar]
|
| 5. |
Piraccini BM, Alessandrini A. Onychomycosis: A review. J Fungi 2015;1:30-43.
[Google Scholar]
|
| 6. |
Piraccini BM, Balestri R, Starace M, Rech G. Nail digital dermoscopy (onychoscopy) in the diagnosis of onychomycosis. J Eur Acad Dermatol Venereol 2013;27:509-13.
[Google Scholar]
|
| 7. |
Jesús-Silva MA, Fernández-Martínez R, Roldán-Marín R, Arenas R. Dermoscopic patterns in patients with a clinical diagnosis of onychomycosis-results of a prospective study including data of potassium hydroxide (KOH) and culture examination. Dermatol Pract Concept 2015;5:39-44.
[Google Scholar]
|
| 8. |
Singal A, Khanna D. Onychomycosis: Diagnosis and management. Indian J Dermatol Venereol Leprol 2011;77:659-72.
[Google Scholar]
|
| 9. |
Nakamura RC, Costa MC. Dermatoscopic findings in the most frequent onychopathies: Descriptive analysis of 500 cases. Int J Dermatol 2012;51:483-5.
[Google Scholar]
|
| 10. |
Yadav TA, Khopkar US. White streaks: Dermoscopic sign of distal lateral subungual onychomycosis. Indian J Dermatol 2016;61:123.
[Google Scholar]
|
| 11. |
Nargis T, Pinto M, Shenoy MM, Hegde S. Dermoscopic features of distal lateral subungual onychomycosis. Indian Dermatol Online J 2018;9:16-9.
[Google Scholar]
|
| 12. |
De Crignis G, Valgas N, Rezende P, Leverone A, Nakamura R. Dermatoscopy of onychomycosis. Int J Dermatol 2014;53:e97-9.
[Google Scholar]
|
| 13. |
Jeelani S, Ahmed QM, Lanker AM, Hassan I, Jeelani N, Fazili T. Histopathological examination of nail clippings using PAS staining (HPE-PAS): Gold standard in diagnosis of Onychomycosis. Mycoses 2015;58:27-32.
[Google Scholar]
|
| 14. |
Amir I, Foering KP, Lee JB. Revisiting office-based direct microscopy for the diagnosis of onychomycosis. J Am Acad Dermatol 2015;72:909-10.
[Google Scholar]
|
| 15. |
Jung MY, Shim JH, Lee JH, Lee JH, Yang JM, Lee DY, et al. Comparison of diagnostic methods for onychomycosis, and proposal of a diagnostic algorithm. Clin Exp Dermatol 2015;40:479-84.
[Google Scholar]
|
| 16. |
Yadav S, Saxena AK, Capoor MR, Ramesh V. Comparison of direct microscopic methods using potassium hydroxide, periodic acid Schiff, and calcofluor white with culture in the diagnosis of onychomycosis. Indian J Dermatol Venereol Leprol 2013;79:242-3.
[Google Scholar]
|
| 17. |
Wilsmann-Theis D, Sareika F, Bieber T, Schmid-Wendtner MH, Wenzel J. New reasons for histopathological nail-clipping examination in the diagnosis of onychomycosis. J Eur Acad Dermatol Venereol 2011;25:235-7.
[Google Scholar]
|
| 18. |
Shenoy MM, Teerthanath S, Karnaker VK, Girisha BS, Krishna Prasad MS, Pinto J. Comparison of potassium hydroxide mount and mycological culture with histopathologic examination using periodic acid-Schiff staining of the nail clippings in the diagnosis of onychomycosis. Indian J Dermatol Venereol Leprol 2008;74:226-9.
[Google Scholar]
|
| 19. |
Karimzadegan-Nia M, Mir-Amin-Mohammadi A, Bouzari N, Firooz A. Comparison of direct smear, culture and histology for the diagnosis of onychomycosis. Australas J Dermatol 2007;48:18-21.
[Google Scholar]
|
| 20. |
Lawry MA, Haneke E, Strobeck K, Martin S, Zimmer B, Romano PS. Methods for diagnosing onychomycosis: A comparative study and review of the literature. Arch Dermatol 2000;136:1112-6.
[Google Scholar]
|
Fulltext Views
8,379
PDF downloads
2,834





