Translate this page into:
Evaluation of anti-gal enzyme-linked immunosorbent assay for the diagnosis of Indian post-kala-azar dermal leishmaniasis
2 Department of Tropical Medicine, School of Tropical Medicine, Kolkata, West Bengal, India
3 Department of Dermatology, Bankura Sammilani Medical College, Government of West Bengal, Kenduadihi, Bankura, West Bengal, India
Correspondence Address:
Sumi Mukhopadhyay
Department of Laboratory Medicine, School of Tropical Medicine, 108 Chittaranjan Avenue, Kolkata - 700 073, West Bengal
India
| How to cite this article: Datta S, Ghosh M, Sarkar S, Saha B, Mukhopadhyay S. Evaluation of anti-gal enzyme-linked immunosorbent assay for the diagnosis of Indian post-kala-azar dermal leishmaniasis. Indian J Dermatol Venereol Leprol 2019;85:578-589 |
Abstract
Background: Elimination of kala azar from India is challenging as there are potential reservoirs of Leishmania donovani in patients with post-kala-azar dermal leishmaniasis (PKDL). The vast repertoire of carbohydrate moieties on L. donovani is known to elicit specific and strong humoral responses in patients with kala azar.
Aim: The present study was undertaken to evaluate the diagnostic performances of anti-gal antibodies using enzyme-linked immunosorbent assay for successful serological diagnosis of PKDL in Indian patients and to differentiate cases of past cured visceral leishmaniasis infections.
Methods: We developed Gal enzyme-linked immunosorbent assay to measure specific anti-gal IgG isotype in the sera of 71 Indian patients with PKDL. The diagnostic efficacy of the newly developed assay was evaluated for precision, sensitivity and accuracy.
Results: Gal2 enzyme-linked immunosorbent assay revealed three-fold increased anti-gal titers in 71 patients with active PKDL compared to controls. Subclass enzyme-linked immunosorbent assay analysis further revealed enhanced IgG2 and IgG3 anti-gal titers in patients with PKDL compared to control subjects. The rank order for specificity and sensitivity for IgG subclasses was IgG3>IgG2>IgG4>IgG1. The area under the curve values of 0.98 and 0.99 were obtained for IgG and IgG3 Gal2 enzyme-linked immunosorbent assays respectively. Overall sensitivity and specificity were 95.7% (95% CI: 88.1–99.1) and 98.1% (95% confidence interval: 90.1–99.9), and 98.5% (95% CI: 92.4–99.9) and 98.1% (95% CI: 90.1–99.9), respectively. Intra-assay coefficient of variation was 1.5% and inter-assay coefficient of variation was 11.7%.
Limitations: The Gal2 enzyme-linked immunosorbent assay needs to be further investigated in mass surveys.
Conclusion: Taken together, anti-gal titers detected through Gal2 enzyme-linked immunosorbent assay can serve as an effective diagnostic tool in disease elimination setting and help in better case management in endemic districts.
Introduction
Visceral leishmaniasis, also known as kala azar, is potentially fatal, if left untreated.[1] Almost 90% of the confirmed cases of visceral leishmaniasis are from India, Bangladesh, Nepal and east Africa, particularly Sudan. In India, it is a major problem in Bihar, Jharkhand, West Bengal and Uttar Pradesh.[2] Post-kala-azar dermal leishmaniasis (PKDL) poses a serious threat to the success of elimination of visceral leishmaniasis. Epidemiologically, global distribution of the condition is fragmentary. Highest number of cases were reported from Sudan and Indian subcontinent, especially Bangladesh with a prevalence up to 16/10,000 population.[3] Main parasitic pathogen causing PKDL is Leishmania donovani, though Leishmania infantum is responsible for sporadic cases in northwestern Iran.[4] In India, there is a prevalence of 4.4–7.8/10,000 in endemic areas of Bihar.[5] Approximate time of acquiring PKDL after visceral leishmaniasis is 0–3 years. The skin lesions are typically characterized by macular and polymorphic lesions on face and body, mostly on sun-exposed areas.[6] The Gangetic plains of Indian subcontinent, including India, Nepal and Bangladesh, are the endemic regions with 52 districts being endemic to kala azar.[7] In India, PKDL occurs in 5–15% of people treated with visceral leishmaniasis.[8] It can also arise without previous history of visceral leishmaniasis.[9] PKDL is the sole reservoir of Leishmania donovani, the causative organism of Indian leishmaniasis, More recently, PKDL has spread to alarming proportions in eastern states like West Bengal.[10],[11]
Accurate diagnosis of visceral leishmaniasis and PKDL is an important component of the visceral leishmaniasis elimination program. Early serodiagnosis of visceral leishmaniasis cases using rK39 strip test is possible. But it is not the best choice, as antibodies can persist after an episode of visceral leishmaniasis. This renders it ineffective for differentiating between cured visceral leishmaniasis and PKDL.[12],[13] Sensitivity of rK39 strip test in detecting macular PKDL is only 73% in India. This is a major concern for mass detection of disease needed for disease elimination.[8] Conventional confirmatory diagnosis with microscopy has a sensitivity of only 33% in macular and 69% in papular PKDL.[8] Other molecular techniques such as polymerase chain reaction/quantitative polymerase chain reaction are available only in some centres and require expert personnel to perform.[14] Clinical diagnosis of the condition is difficult and requires an experienced clinician. Previously, we reported that polyethylene glycol enzyme-linked immunosorbent assay for detecting antibody bound to circulating immune complexes has 60–80% sensitivity in Indian PKDL.[15] Therefore, there is an existing need for an effective and reliable assay for the diagnosis of PKDL.
Human serum constitutes antibodies against immunogenic carbohydrates and provides a rich reservoir for potential biomarkers of many diseases. Around 1% of the human IgG consists of anti-carbohydrate antibodies of anti-gal nature.[16] Carbohydrate epitopes in parasitic infections including leishmaniasis are known to elicit profound humoral response in the host. Anti-gal antibodies have been reported to be important for developing protective immunity against T. cruzi in Chagas disease. Its titer is 10–16 fold more in patients with Chagas disease compared with healthy individuals.[17],[18] Anti-gal antibody levels were significantly increased in 66% of patients with chronic Chagasic cardiomyopathy.[19] Patients with different clinical forms of leishmaniasis including visceral leishmaniasis have elevated galactosyl alpha (1-3) galactose antibodies.[20] Studies have confirmed the presence of high levels of anti α-galactosyl antibodies in dermal parasitic diseases such as old world cutaneous leishmaniasis.[21] Simple tools such as enzyme-linked immunosorbent assay have become a rapid and effective way of serodiagnosis. Studies have also established that anti-gal enzyme-linked immunosorbent assay has a sensitivity of 89% in patients with diffuse cutaneous leishmaniasis and 84% in patients with localized cutaneous leishmaniasis.[22] IgG enzyme-linked immunosorbent assay showed sensitivity of 96% for L. major and 91% for L. tropica infections and was equivalent to restriction fragment length polymorphism-polymerase chain reaction analysis of parasite ITS1 gene.[21] The present study attempts to evaluate the potential of anti-gal antibodies for accurate and rapid diagnosis of Indian patients with PKDL.
Methods
Study population and design
Patients with PKDL from block levels in different districts were enrolled at School of Tropical Medicine, Kolkata. The patients were recruited into a case–control study, who were mostly natives of endemic districts of West Bengal. They underwent physical examination by clinicians. RK39 strip test (using Kala Azar ™ Rapid Test InBios International, Seattle, WA, USA) and parasitological confirmation by microscopy were done. After explanation of the purpose of study, written informed consent was obtained from all subjects for taking clinical images and collecting venous blood. Sera samples were collected from 71 patients with PKDL. Clinico-epidemiological parameters such as age, sex and medication history were carefully recorded. Twenty eight patients with other skin diseases such as leprosy and vitiligo were enrolled as disease control subjects. As endemic controls, we tested sera from 26 volunteers, who were apparently healthy living in the endemic zones. Eleven rK39 positive patients cured from visceral leishmaniasis were also enrolled into the study. Enrolled patients with PKDL were treated with liposomal amphotericin B (twice weekly at 5 mg/kg body weight for 3 weeks)/miltefosine (50 mg/kg body weight for 12 weeks for adults <25 kg and 100 mg/kg body weight for adults> 25 kgs) as per national health policy guidelines.[23]
Ethical statement
The ethical aspects were reviewed and approved by the Institutional Clinical Research Ethics Committee of School of Tropical Medicine, Kolkata (CREC-STM).
Biological samples
Peripheral blood from all consenting subjects was collected through venepuncture. For separation of serum, 2 ml of venous blood was collected in vial lacking anticoagulant. Continuous cold chain was maintained for cryopreservation until further use.
Leishmania donovani antigen enzyme-linked immunosorbent assay
To determine antileishmanial IgG antibody titers, L. donovani antigen enzyme-linked immunosorbent assay was performed.[24]L. donovani antigen obtained from membrane lysis of L. donovani strain MHOM/IN/83/AG83 in phosphate buffer (0.02 M, pH 7.2) was coated at optimal concentration (1 μg/100 μl/well). After blocking nonspecific sites, sera from enrolled subjects (1:500; 100 μl/well) were incubated for 2 h at 4°C in microtiter well plates (Nalge NuncIntl., USA). Subsequently, horse radish peroxidase-conjugated antihuman monoclonal IgG (1:15,000) was added to measure the levels of IgG isotype of antileishmanial antibodies with tetramethylbenzidine. Optical density was measured at 450 nm. The reaction was stopped after 5 min with 2 N H2 SO4.
Gal enzyme-linked immunosorbent assay
For detection of anti-gal antibodies, two heavily glycosylated proteins, namely porcine stomach mucin (Sigma, USA) and asialofetuin (Sigma, USA), were selected and used as coating antigens (1 μg/100 μl/well). Both the glycosylated proteins have been used as purified forms from animal origin containing galactose bearing terminal residues. Microtiter well plates (Nalge NuncIntl., USA) were coated with 0.01 mg/ml of capture glycoproteinantigen (porcine stomach mucin or asialofetuin) in 0.05 M carbonate buffer (pH 9.6) and kept overnight at 4°C. After washing, binding of sera was measured with mouse antihuman IgG peroxidase-conjugated antibody (Sigma, USA) diluted to 1:15,000. For subclass antibody determination, binding of sera was measured with peroxidase-conjugated antihuman IgG1 or IgG2 or IgG3 or IgG4 (Invitrogen, USA) diluted to 1:1000. Finally, the plates were washed and incubated with tetramethylbenzidine as substrate. The reaction was stopped after 5 min with 50 μl of 2N H2 SO4. Optical density was measured at 450 nm on a microplate reader (Erba Lisa Scan II, Germany). Humoral responses in terms of antibody titers were measured in optical density values.
Statistical analysis
Data were analyzed using Prism 6.0 software (GraphPad, USA). Differences among means were analyzed by one-way analysis of variance and post-hoc comparison with Bonferroni correction to determine statistical significance. P < 0.05 was considered significant. Sensitivity and specificity for each enzyme-linked immunosorbent assay were calculated using the equations: sensitivity = true positive/(true positive + false negative) × 100% and specificity = true negative/(true negative + false positive) × 100%. Positive predictive value and negative predictive value were calculated by using the equations: positive predictive value = true positive/(true positive + false positive) × 100% and negative predictive value = true negative/(true negative + false negative) × 100%. Validity of enzyme-linked immunosorbent assay was calculated using coefficient of variation when the samples were run in duplicates. Intra-assay and inter-assay coefficient of variation were estimated as percentages (CV%). The cut-off was calculated using Youden's index method.
Results
The study population consisted of 71 confirmed cases of PKDL (35 males and 36 females) enrolled from Malda and Birbhum districts of West Bengal during 2015–2017. Median age of the study population was 19 years (range: 5 to 60 years; mean = 23.2 years) [Table - 1].
There were 98.5% (n = 70) of subjects, who had past history of visceral leishmaniasis. Majority were village farmers from lower socio-economic strata.
Among the study subjects, 74.6% (53/71) patients with PKDL and previous history of visceral leishmaniasis episodes had history of being treated with sodium stibogluconate. Median time of development of PKDL was seven years for patients treated with sodium stibogluconate, whereas it was four years in patients with visceral leishmaniasis treated with miltefosine and three years in patients with previous visceral leishmaniasis treated with liposomal amphotericin B, respectively [Table - 2].

Status of IgG antibodies against Leishmania donovani antigen in patients with PKDL
The evaluation of anti-leishmanial IgG antibodies was done in the sera of patients with PKDL along with patients with other skin diseases and healthy individuals. L. donovani antigen-based enzyme-linked immunosorbent assay demonstrated increased antibody IgG responses in patients with PKDL compared to control groups. The overall sensitivity was 91.5% (95% confidence interval: 82.5–96.8). Only two patients presenting with other skin diseases showed test positivity. There was increased anti-leishmanial antibody titers in terms of “mean ± SEM” in sera of patients with PKDL (0.688 ± 0.035) as compared with antibody titers of patients with diseases such as leprosy and vitiligo as disease controls (0.252 ± 0.009) and healthy individuals as endemic controls (0.241 ± 0.005).
Seropositivity of anti-gal IgG antibodies in patients with PKDL
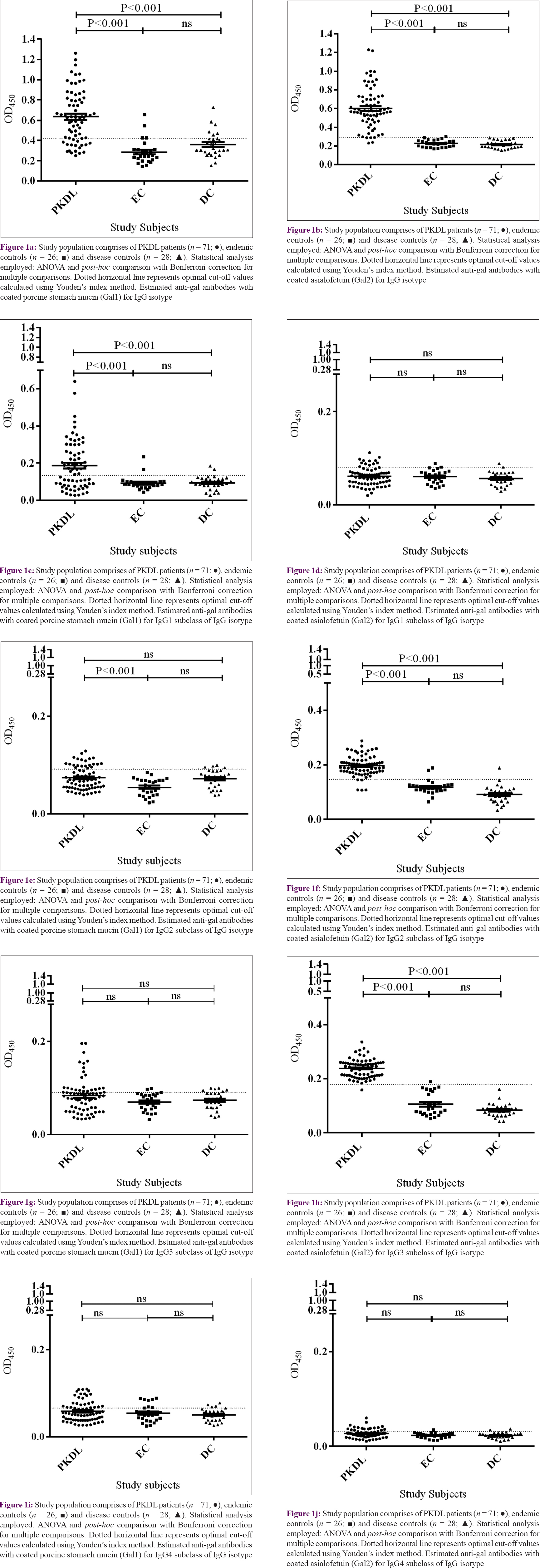
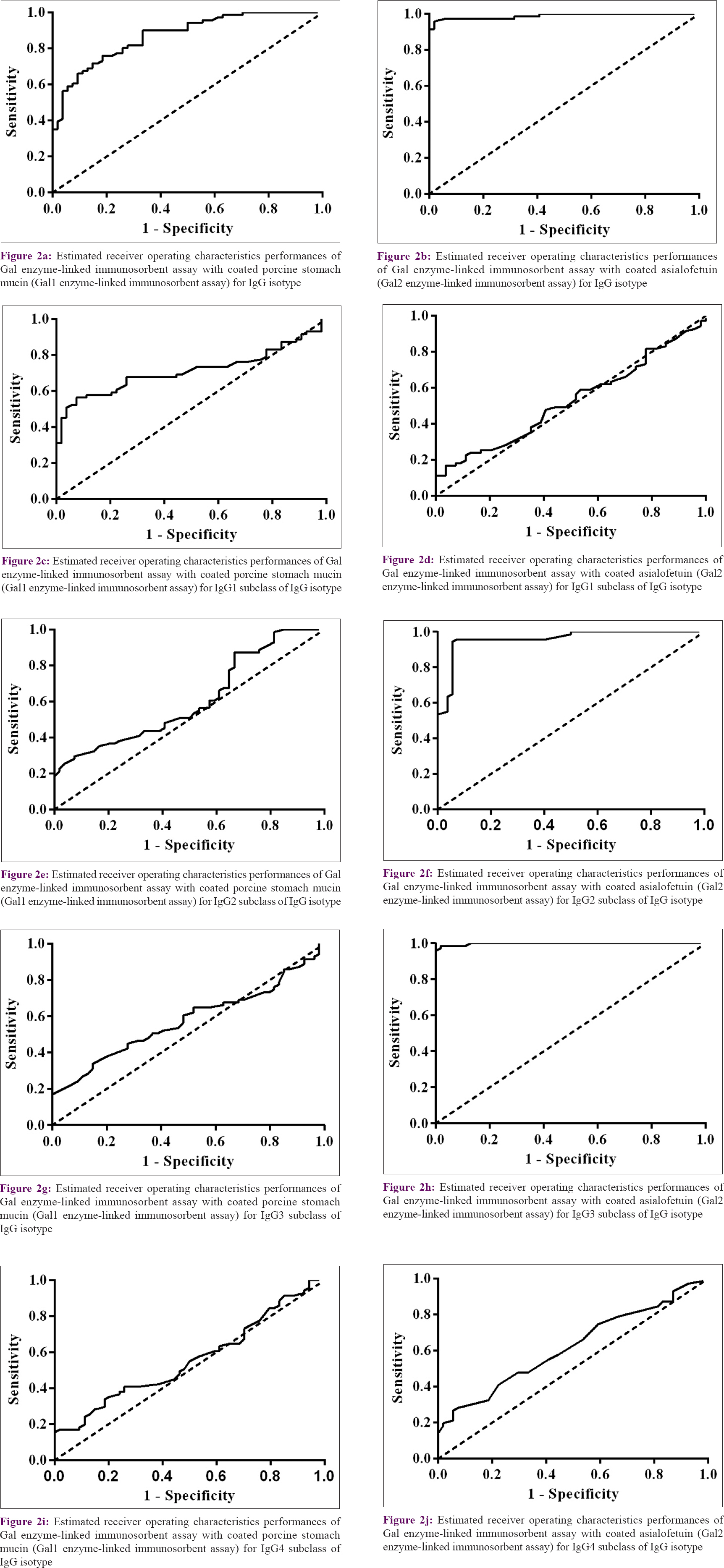
We compared the performances of two enzyme-linked immunosorbent assay tests using two different heavily glycosylated proteins as coating materials. The enzyme-linked immunosorbent assay using porcine stomach mucin was termed as Gal1 enzyme-linked immunosorbent assay and the one using asialofetuin was termed as Gal2 enzyme-linked immunosorbent assay. All rK39 positive patients were tested with both enzyme-linked immunosorbent assays. Sensitivity of IgG Gal2 enzyme-linked immunosorbent assay was 95.7% (95% confidence interval: 88.1–99.1) and IgG Gal1 enzyme-linked immunosorbent assay was 76.06% (95% CI: 64.4–85.3). Specificity of IgG Gal1 enzyme-linked immunosorbent assay was 81.4% (95% CI: 68.5–90.7) and IgG Gal2 enzyme-linked immunosorbent assay was 98.1% (95% CI: 90.1–99.9). Twenty-eight patients presenting with various skin diseases were analyzed. None had seropositivity with Gal2 enzyme-linked immunosorbent assay, whereas six patients showed seropositivity with Gal1 enzyme-linked immunosorbent assay. Anti-gal antibody titers for porcine stomach mucin-coated enzyme-linked immunosorbent assay in terms of “mean ± SEM” were higher (0.635 ± 0.030) in the sera of patients with PKDL compared to patients with diseases such as leprosy and vitiligo as disease controls (0.358 ± 0.023) and endemic controls (0.285 ± 0.023). Similarly, sera IgG titers (0.602 ± 0.025) of patients with PKDL were higher for asialofetuin-coated enzyme-linked immunosorbent assay compared to disease controls (0.214 ± 0.007) and endemic controls (0.225 ± 0.007). Analysis of variance showed significant differences (P < 0.001) among the study subjects [Figure - 1]a and [Figure - 1]b. Diagnostic performances of both assays were tested. The area under the curve value of IgG Gal1 enzyme-linked immunosorbent assay was 0.87, compared to 0.98 for IgG Gal2 enzyme-linked immunosorbent assay [Figure - 2]a,[Figure - 2]b,[Figure - 2]c,[Figure - 2]d,[Figure - 2]e,[Figure - 2]f,[Figure - 2]g,[Figure - 2]h,[Figure - 2]i,[Figure - 2]j.
 |
| Figure 1: |
 |
| Figure 2: |
IgG1, IgG2, IgG3 and IgG4 enzyme-linked immunosorbent assays were performed for determining the subclass titers. There was a preponderance of IgG and IgG3 antibodies. IgG2 and IgG3 Gal2 enzyme-linked immunosorbent assays showed significant differences between patients with PKDL and the control groups. IgG1 and IgG titers on Gal1 enzyme-linked immunosorbent assays were significantly different with respect to both endemic and disease control groups [Figure - 1]c,[Figure - 1]d,[Figure - 1]e,[Figure - 1]f,[Figure - 1]g,[Figure - 1]h,[Figure - 1]i,[Figure - 1]j.
We also analyzed the presence of anti-gal antibodies, as found in different lesion types of patients with PKDL. There were four types of lesions (i) diffuse macular, (ii) macular, (iii) papular and (iv) polymorphic. Polymorphic PKDL constituted 38% (27/71) of the enrolled cases. Seropositivity of 88.8% (24/27) was obtained with IgG Gal1 enzyme-linked immunosorbent assay and 96.2% (26/27) with IgG Gal2 enzyme-linked immunosorbent assay [Table - 3]. Polymorphic forms of PKDL showed almost three-fold enhanced titers than controls in the endemic districts. 46.4% (33/71) of patients had macular PKDL. 32 out of 33 cases (96.9%) were detected positive with IgG Gal2 enzyme-linked immunosorbent assay, whereas Gal1 enzyme-linked immunosorbent assay could detect 23 out of 33 cases(69.6%). Three out of five (60%) papular cases were detected with IgG Gal1 enzyme-linked immunosorbent assay, whereas all five (100%) cases could be detected by IgG Gal2 enzyme-linked immunosorbent assay. Out of six patients with diffuse macular PKDL, five (83.3%) were detected with IgG Gal2 enzyme-linked immunosorbent assay, whereas four (66.6%) were detected with IgG Gal1 enzyme-linked immunosorbent assay [Table - 3]. The coating antigen asialofetuin used in Gal2 enzyme-linked immunosorbent assay was better studied as glycoprotein bearing predominant terminal galactose residues than porcine stomach mucin.

IgG Gal-enzyme-linked immunosorbent assays were validated by inter-assay coefficient of variation % (CV%) and intra-assay coefficient of variation % (CV%) as measures of reproducibility. IgG Gal2 enzyme-linked immunosorbent assay had intra-assay CV% of 1.5% and inter-assay CV% of 11.7%. The corresponding values were 1.4% and 21.2%, respectively, when samples were repeated in duplicates in batches.
Comparative evaluation of anti-gal IgG antibodies in patients with cured visceral leishmaniasis and fresh PKDL
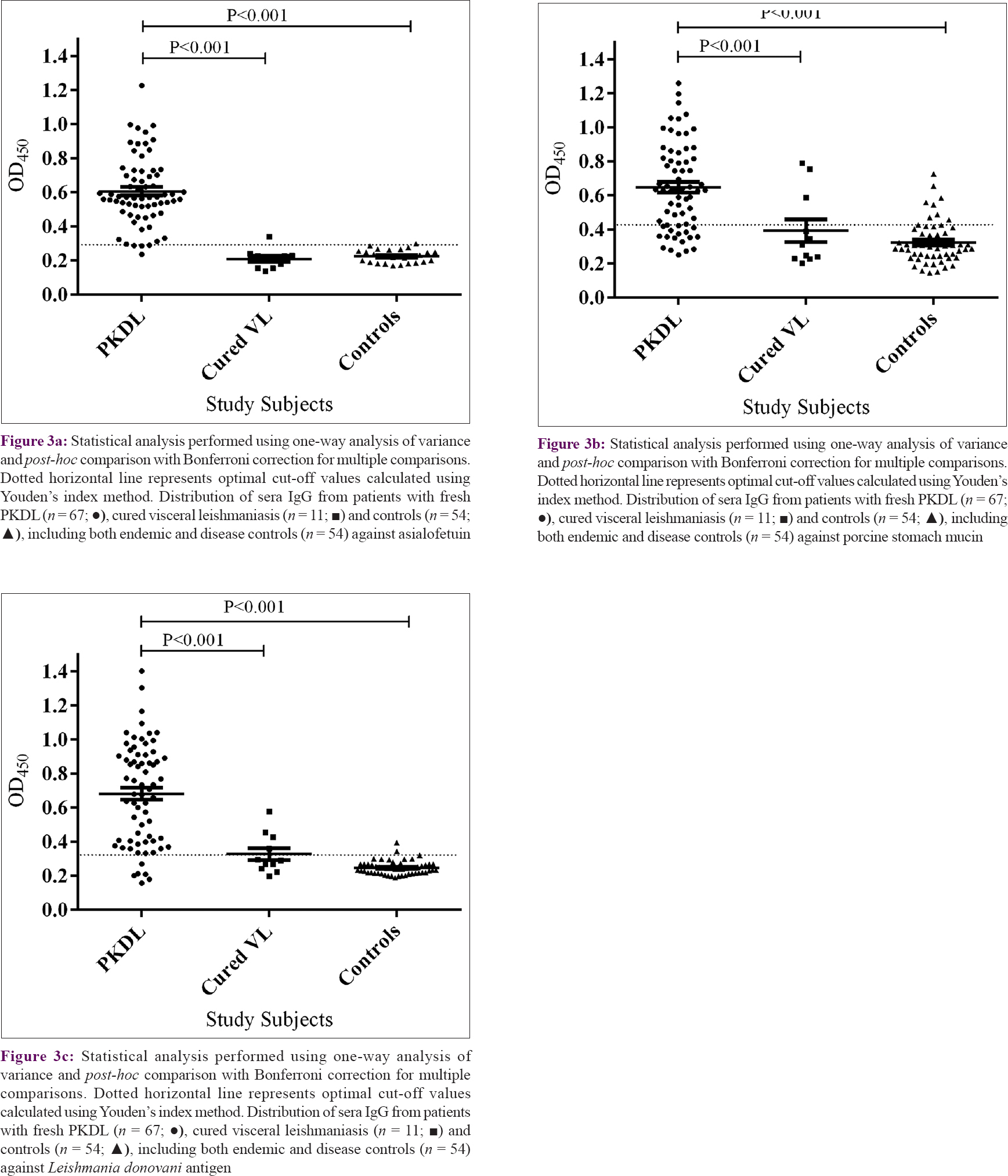
There was elevated anti-gal antibody titers for IgG Gal2 enzyme-linked immunosorbent assay in sera of patients with fresh PKDL (mean = 0.606; SEM = 0.024) compared with patients cured from visceral leishmaniasis (0.209 ± 0.016) (P < 0.001). Similarly, sera IgG titers detected by IgG Gal1 enzyme-linked immunosorbent assay of fresh patients with PKDL (0.646 ± 0.031) were significantly higher (P < 0.001) compared to patients cured from visceral leishmaniasis (0.392 ± 0.065). L. donovani antigen IgG enzyme-linked immunosorbent assay also showed significantly higher anti-leishmanial antibody titers in sera of fresh patients with PKDL (0.682 ± 0.036) compared to patients cured from visceral leishmaniasis (0.327 ± 0.034) (P < 0.001), though four cases of cured visceral leishmaniasis had persistent anti-leishmanial antibodies [Figure - 3]a,[Figure - 3]b,[Figure - 3]c. However, IgG Gal2 enzyme-linked immunosorbent assay could successfully identify all except one of the cured cases as true negative. Anti-gal antibodies were negative in cured visceral leishmaniasis cases even when anti-leishmanial antibodies were persisting.
 |
| Figure 3: |
Discussion
In India, transmission of visceral leishmaniasis infection is anthroponotic where unattended cases of active PKDL serve as a durable reservoir of L. donovani, particularly during the inter-epidemic period.[14],[25] Thus, accurate diagnosis of PKDL is an important component of visceral leishmaniasis elimination program. Current diagnostic modalities of PKDL, particularly the macular forms, is limited by low sensitivities and specificities. In this paper we report development of a PKDL-specific diagnostic assay with improved sensitivity and specificity.
Most of the enrolled patients with Indian PKDL showed hypopigmented macular lesions (39/71 = 54.9%) as the prominent clinical manifestation.
Studies have demonstrated that there was raised anti-Gal IgG antibody titers in humans against Galα1–2Galβ bearing glycoconjugate on Leishmania major- as well as Galα1–3Galβ glycoconjugates present on Trypanosoma cruzi.[26] Sensitivity of anti-Gal (Galα1–3Man) antibodies in detecting cutaneous leishmaniasis was 69–89%, compared to 44–62% for trypanosomatid infection.[22] Previous studies have also demonstrated presence of high titers of disease-specific anti-O-acetylated sialic acid antibodies in sera of Indian patients with visceral leishmaniasis.[24] Recent studies from our laboratory revealed the status of carbohydrates in circulating immune complexes in patients with PKDL; however, the nature of these antibodies remains unclear.[27] The present study reports the diagnostic utility of anti-gal antibodies for early diagnosis of patients presenting with different clinical types of PKDL. Saha et al. have demonstrated the diagnostic potential of anti-leishmanial IgG3 antibody titers by the use of subclass L. donovani crude antigen-based enzyme-linked immunosorbent assays in Indian patients with PKDL.[28] Our study with Gal2 enzyme-linked immunosorbent assay revealed a 2.26-fold higher titer of IgG3 anti-gal levels compared to endemic healthy controls and 2.83-fold higher titers compared to patients with diseases such as leprosy and vitiligo [Figure - 1]h. In addition, our results showed that the IgG Gal2 enzyme-linked immunosorbent assay had very good agreement with rK39 strip test (κ = 0.84), whereas IgG Gal1 enzyme-linked immunosorbent assay had fair agreement (κ = 0.316). Thus, IgG Gal2 enzyme-linked immunosorbent assay has both high sensitivity and specificity. Porcine stomach mucin, the coating proteins for Gal1 enzyme-linked immunosorbent assay, is a rich source of different types of oligosaccharides, but is relatively less abundant in terminal galactose residues than asialofetuin, the coating protein of Gal2 enzyme-linked immunosorbent assay.[29],[30] Therefore, anti-gal antibody capturing is less in Gal1 enzyme-linked immunosorbent assay compared with Gal2 enzyme-linked immunosorbent assay.
Serologically, it is difficult to distinguish treated cases of visceral leishmaniasis from fresh cases of PKDL, as there is persistence of anti-leishmanial antibody titers even after cure.[31]L. donovani antigen-based enzyme-linked immunosorbent assay showed persistence of anti-leishmanial antibodies in both groups [Figure - 3]c. Interestingly, Gal enzyme-linked immunosorbent assay was found to possess a discriminatory potential for monitoring cured visceral leishmaniasis from fresh cases of PKDL. There was 2.3-fold higher anti-gal IgG titers in sera of fresh patients with PKDL as compared with cured cases of visceral leishmaniasis in IgG Gal1 enzyme-linked immunosorbent assay, whereas there was a 2.9-fold increase in anti-gal IgG sera titers in cured visceral leishmaniasis cases in IgG Gal2 enzyme-linked immunosorbent assay [Figure - 3]a and [Figure - 3]b. This indicates the potential of anti-gal antibodies to differentiate cured cases from fresh cases of PKDL, in whom all the subjects showed rK39 positivity. In addition, 10/11 (90.9%) and 8/11 cases (72.7%) of cured visceral leishmaniasis were identified as true negative with IgG Gal2 enzyme-linked immunosorbent assay and IgG Gal1 enzyme-linked immunosorbent assay, respectively. But, L. donovani antigen-based enzyme-linked immunosorbent assay showed relatively high antileishmanial antibody titers in cured visceral leishmaniasis and could not differentiate them from fresh PKDL.
The positive predictive value of IgG Gal2 enzyme-linked immunosorbent assay (68/68 = 100%) was higher than that of IgG Gal1 enzyme-linked immunosorbent assay (54/64 = 84.3%). The higher negative predictive values obtained with IgG Gal2 enzyme-linked immunosorbent assay (54/57 = 94.7%) in comparison to IgG Gal1 enzyme-linked immunosorbent assay (44/61 = 72.1%), pointed to the better potential of the former test to predict negative test controls. Whereas, both the proportions of true positives (70/71 = 98.5%) and true negatives (53/54 = 98.1%) as predicted with the IgG3 Gal2 enzyme-linked immunosorbent assay, showed high accuracy among all subclasses employed.
The area under the curve values of 0.98 and 0.99 for IgG Gal2 and IgG3 Gal2 enzyme-linked immunosorbent assays, respectively, showcases the excellent diagnostic utility of anti-gal antibodies in PKDL [Figures 2a-j], whereas L. donovani antigen-based IgG enzyme-linked immunosorbent assay showed area under the curve value of 0.92 (graph not shown). Therefore, anti-gal IgG and IgG3 antibodies in sera of patients from endemic areas have immense diagnostic potential in detecting cases of PKDL. Moreover, the anti-gal IgG enzyme-linked immunosorbent assay test could efficiently differentiate cured visceral leishmaniasis from fresh PKDL.
One limitation of the study is that the anti-gal enzyme-linked immunosorbent assay was not evaluated in the sera of cured PKDL and active visceral leishmaniasis. Furthermore, this test needs to be applied on mass field surveys for validating the differential diagnosis of PKDL in the endemic settings.
We believe that anti-gal antibodies could be used as effective screening markers for accurate, effective and rapid diagnosis of PKDL. Gal enzyme-linked immunosorbent assays could be used for systematic screening of suspected subjects in endemic areas and could be useful to differentiate cured cases of leishmaniasis from new cases of PKDL. The enzyme-linked immunosorbent assay is very easy to use and is cost-effective. The anti-gal estimation through the novel gal enzyme-linked immunosorbent assay appears to be an important diagnostic tool for improved diagnosis of Indian PKDL. This assay has the potential to differentiate cured visceral leishmaniasis from fresh cases of PKDL and could markedly reduce the rate of false positivity and improve specificity of the assay.
Conclusion
To conclude, anti-gal titers estimated by Gal2 enzyme-linked immunosorbent assay can serve as an effective diagnostic tool thereby helping the efforts to eliminate leishmaniasis. It could also help in better case management in endemic districts. Moreover, it has the potential to differentiate PKDL from past leishmaniasis infection.
Acknowledgements
We are thankful to all the patients and their family members for their cooperation. S. Datta is the recipient of Senior Research Fellowship from the University Grants Commission, Government of India.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the forms, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
This work was supported by a grant from the Indian Council of Medical Research (ICMR) (grant number: 5/8-7(96) V-2011-ECD-II) and Science and Engineering Research Board (grant number: YSS/2014/000934), Government of India, New Delhi.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Lal CS, Verma RB, Verma N, Siddiqui NA, Rabidas VN, Pandey K, et al. Hypertriglyceridemia: A possible diagnostic marker of disease severity in visceral leishmaniasis. Infection 2016;44:39-45.
[Google Scholar]
|
| 2. |
Bhunia GS, Kesari S, Jeyaram A, Kumar V, Das P. Influence of topography on the endemicity of kala-azar: A study based on remote sensing and geographical information system. Geospat Health 2010;4:155-65.
[Google Scholar]
|
| 3. |
Mondal D, Nasrin KN, Huda MM, Kabir M, Hossain MS, Kroeger A, et al. Enhanced case detection and improved diagnosis of PKDL in a kala-azar-endemic area of Bangladesh. PLoS Negl Trop Dis 2010;4. pii: e832.
[Google Scholar]
|
| 4. |
Badirzadeh A, Mohebali M, Ghasemian M, Amini H, Zarei Z, Akhoundi B, et al. Cutaneous and post kala-azar dermal leishmaniasis caused by Leishmania infantum in endemic areas of visceral leishmaniasis, Northwestern Iran 2002-2011: A case series. Pathog Glob Health 2013;107:194-7.
[Google Scholar]
|
| 5. |
Singh RP, Picado A, Alam S, Hasker E, Singh SP, Ostyn B, et al. Post-kala-azar dermal leishmaniasis in visceral leishmaniasis-endemic communities in Bihar, India. Trop Med Int Health 2012;17:1345-8.
[Google Scholar]
|
| 6. |
Zijlstra EE, Alvar J. The post kala-azar dermal leishmaniasis (PKDL) atlas. Lancet Infect Dis 2013;3:87-98.
[Google Scholar]
|
| 7. |
Zijlstra EE, Alves F, Rijal S, Arana B, Alvar J. Post-kala-azar dermal leishmaniasis in the Indian subcontinent: A threat to the South-East Asia region kala-azar elimination programme. PLoS Negl Trop Dis 2017;11:e0005877.
[Google Scholar]
|
| 8. |
Salotra P, Singh R. Challenges in the diagnosis of post kala-azar dermal leishmaniasis. Indian J Med Res 2006;123:295-310.
[Google Scholar]
|
| 9. |
Das VN, Ranjan A, Pandey K, Singh D, Verma N, Das S, et al. Clinical epidemiologic profile of a cohort of post-kala-azar dermal leishmaniasis patients in Bihar, India. Am J Trop Med Hyg 2012;86:959-61.
[Google Scholar]
|
| 10. |
Addy M, Nandy A. Ten years of kala-azar in West Bengal, part I. Did post-kala-azar dermal leishmaniasis initiate the outbreak in 24-parganas? Bull World Health Organ 1992;70:341-6.
[Google Scholar]
|
| 11. |
Singh R, Kumar D, Ramesh V, Negi NS, Singh S, Salotra P. Visceral leishmaniasis, or kala azar (KA): High incidence of refractoriness to antimony is contributed by anthroponotic transmission via post-KA dermal leishmaniasis. J Infect Dis 2006;194:302-6.
[Google Scholar]
|
| 12. |
Haldar JP, Saha KC, Ghose AC. Serological profiles in Indian post kala-azar dermal leishmaniasis. Trans R Soc Trop Med Hyg 1981;75:514-7.
[Google Scholar]
|
| 13. |
El Harith A, Chowdhury S, Al-Masum A, Semião-Santos S, Das PK, Akhter S, et al. Reactivity of various leishmanial antigens in a direct agglutination test and their value in differentiating post-kala azar dermal leishmaniasis from leprosy and other skin conditions. J Med Microbiol 1996;44:141-6.
[Google Scholar]
|
| 14. |
Ramesh V, Kaushal H, Mishra AK, Singh R, Salotra P. Clinico-epidemiological analysis of post kala-azar dermal leishmaniasis (PKDL) cases in India over last two decades: A hospital based retrospective study. BMC Public Health 2015;15:1092.
[Google Scholar]
|
| 15. |
Datta S, Modak D, Sarkar S, Saha B, Mukhopadhyay S. Identification and glycobiological characterization of circulating immune complexes in patients with visceral leishmaniasis and post-kala-azar dermal leishmaniasis. Indian J Exp Biol 2015;53:321-8.
[Google Scholar]
|
| 16. |
Mosedale DE, Chauhan A, Schofield PM, Grainger DJ. A pattern of anti-carbohydrate antibody responses present in patients with advanced atherosclerosis. J Immunol Methods 2006;309:182-91.
[Google Scholar]
|
| 17. |
González J, Ramírez C, Seguel X, Gutiérrez B, Manque P, Porcile P, et al. Levels of anti-gal antibodies in persons infected and non-infected with Trypanosoma Cruzi. Probably induced by bacteria and by the parasite. Bol Chil Parasitol 1995;50:3-9.
[Google Scholar]
|
| 18. |
Avila JL, Rojas M, Galili U. Immunogenic gal alpha 1–3Gal carbohydrate epitopes are present on pathogenic American Trypanosoma and Leishmania. J Immunol 1989;142:2828-34.
[Google Scholar]
|
| 19. |
Avila JL, Rojas M, Velazquez-Avila G. Characterization of a natural human antibody with anti-galactosyl (alpha 1-2) galactose specificity that is present at high titers in chronic Trypanosoma cruzi infection. Am J Trop Med Hyg 1992;47:413-21.
[Google Scholar]
|
| 20. |
Bretaña A, Avila JL, Contreras-Bretaña M, Tapia FJ. American leishmania spp. and Trypanosoma cruzi: Galactosyl alpha (1-3) galactose epitope localization by colloidal gold immunocytochemistry and lectin cytochemistry. Exp Parasitol 1992;74:27-37.
[Google Scholar]
|
| 21. |
Al-Salem WS, Ferreira DM, Dyer NA, Alyamani EJ, Balghonaim SM, Al-Mehna AY, et al. Detection of high levels of anti-α-galactosyl antibodies in sera of patients with old world cutaneous leishmaniasis: A possible tool for diagnosis and biomarker for cure in an elimination setting. Parasitology 2014;141:1898-903.
[Google Scholar]
|
| 22. |
Avila JL, Rojas M. A galactosyl (alpha 1-3) mannose epitope on phospholipids of Leishmania mexicana and L. braziliensis is recognized by trypanosomatid-infected human sera. J Clin Microbiol 1990;28:1530-7.
[Google Scholar]
|
| 23. |
http://nvbdcp.gov.in/Doc/opertional-guideline-KA-2015.pdf
[Google Scholar]
|
| 24. |
Bandyopadhyay S, Chatterjee M, Pal S, Waller RF, Sundar S, McConville MJ, et al. Purification, characterization of O-acetylated sialoglycoconjugates-specific IgM, and development of an enzyme-linked immunosorbent assay for diagnosis and follow-up of Indian visceral leishmaniasis patients. Diagn Microbiol Infect Dis 2004;50:15-24.
[Google Scholar]
|
| 25. |
Ganguly S, Saha P, Chatterjee M, Roy S, Ghosh TK, Guha SK, et al. PKDL – A silent parasite pool for transmission of leishmaniasis in kala-azar endemic areas of Malda district, West Bengal, India. PLoS Negl Trop Dis 2015;9:e0004138.
[Google Scholar]
|
| 26. |
Schneider C, Smith DF, Cummings RD, Boligan KF, Hamilton RG, Bochner BS, et al. The human IgG anti-carbohydrate repertoire exhibits a universal architecture and contains specificity for microbial attachment sites. Sci Transl Med 2015;7:269ra1.
[Google Scholar]
|
| 27. |
Jaiswal P, Datta S, Sardar B, Chaudhuri SJ, Maji D, Ghosh M, et al. Glycoproteins in circulating immune complexes are biomarkers of patients with Indian PKDL: A study from endemic districts of West Bengal, India. PLoS One 2018;13:e0192302.
[Google Scholar]
|
| 28. |
Saha S, Mazumdar T, Anam K, Ravindran R, Bairagi B, Saha B, et al. Leishmania promastigote membrane antigen-based enzyme-linked immunosorbent assay and immunoblotting for differential diagnosis of Indian post-kala-azar dermal leishmaniasis. J Clin Microbiol 2005;43:1269-77.
[Google Scholar]
|
| 29. |
Nordman H, Davies JR, Herrmann A, Karlsson NG, Hansson GC, Carlstedt I. Mucus glycoproteins from pig gastric mucosa: Identification of different mucin populations from the surface epithelium. Biochem J 1997;326 (Pt 3):903-10.
[Google Scholar]
|
| 30. |
Yet MG, Chin CC, Wold F. The covalent structure of individual N-linked glycopeptides from ovomucoid and asialofetuin. J Biol Chem 1988;263:111-7.
[Google Scholar]
|
| 31. |
Desjeux P, Ghosh RS, Dhalaria P, Strub-Wourgaft N, Zijlstra EE. Report of the post kala-azar dermal leishmaniasis (PKDL) consortium meeting, New Delhi, India, 27-29 June 2012. Parasit Vectors 2013;6:196.
[Google Scholar]
|
Fulltext Views
2,911
PDF downloads
1,155





