Translate this page into:
Study of the relation between two common cyclooxygenase 2 gene polymorphisms with risk of developing and subtypes of vitiligo in Egyptian patients
2 Department of Dermatology and Venereology, National Research Centre, Giza, Egypt
3 Department of Medical Molecular Genetics, National Research Centre, Giza, Egypt
Correspondence Address:
Samar Abdallah M Salem
Department of Dermatology, Venereology and Andrology, Faculty of Medicine, Ain Shams University, Abbasseya Square, Cairo
Egypt
| How to cite this article: M Salem SA, Aly DG, Amr KS, Abdel-Hamid MF. Study of the relation between two common cyclooxygenase 2 gene polymorphisms with risk of developing and subtypes of vitiligo in Egyptian patients. Indian J Dermatol Venereol Leprol 2018;84:696-700 |
Abstract
Background/Purpose: Genetic factors play an important role in the pathogenesis of vitiligo. Cyclooxygenase 2 (COX2) gene induced by ultraviolet radiation controls the synthesis of prostaglandins, which are are found to be beneficial in treating vitiligo. COX2 gene polymorphism has been previously evaluated in Chinese population. We aimed to study the relation between two common COX2 gene polymorphisms with vitiligo and its subtypes amongEgyptian patients.
Patients and Methods: This study included 200 participants (100 vitiligo patients and 100 healthy controls). COX2-765G/C and -1195A/G gene polymorphism was studied by restriction fragment length polymorphism polymerase chain reaction analysis and the results were compared between the two groups and among different subtypes of vitiligo.
Results: Frequency of COX2-1195 AA, AG, GG genotypes showed no significant association among patients with vitiligo (P = 0.626, 0.321, 0.08, respectively); those with generalized vitiligo (P = 0.739, 0.291, 0.101, respectively) and those with segmental vitiligo (P = 0.410, 1.00, 0.676, respectively) compared to the control group. Regarding COX2-765G/C genotypes, GG genotype was more frequent among patients with vitiligo [84 (84%)] compared to controls [63 (63%)] (P = 0.001). GC genotype was significantly less frequent [15 (15%)] among patients compared to controls [32 (32%)] (P = 0.005). Generalized and segmental types of vitiligo also showed no significant difference in the frequency of COX2-765G/C genotypes compared with controls.
Limitations: Being a pilot study, a relatively small number of participants were included.
Conclusion: COX2-1195A/G gene polymorphism is not associated with the risk of developing vitiligo or with vitiligo subtypes. COX2-765 GG genotype is associated with vitiligo, especially of the generalized type.
Introduction
Vitiligo is a common acquired depigmentation disorder of the skin manifested clinically as white macules.[1] Clinical presentation includes segmental, non-segmental, and mixed vitiligo.[2] Though its exact pathogenesis is not clearly known, vitiligo is considered as a multifactorial disease 3 in which genetic and environmental factors play a role.[4]
According to genetic hypothesis, vitiligo has a multigenic inheritance. Several attempts have been made to identify a risk group pattern for vitiligo by genetic analyses.[5]
Prostaglandin E2 is synthesized in skin. It causes melanocyte proliferation; influences responsiveness of melanocytes to neuronal stimuli; induces changes in melanocytes necessary for transfer of melanosomes to surrounding keratinocytes, stimulates tyrosinase activity;[6] and enhances basic fibroblast growth factor mRNA expression[7] (the derived peptide of which is effective locally in vitiligo).[8] Oxidative stress, known to cause melanocyte destruction in vitiligo, results in a decrease in prostaglandin E2.[9] Prostaglandin E2 also inhibits apoptosis, stimulates inflammatory response, and promotes immunosuppression.[10] Topical prostaglandin E2 is a promising therapy for localized stable vitiligo.[11],[12]
Cyclooxygenases are key enzymes that mediate the conversion of free arachidonic acid into prostaglandin H2 and other eicosanoids.[13],[14],[15] There are two major cyclooxygenase (COX) isoforms, COX1 and COX2. COX1 is constitutively expressed in most cell types, while COX2 is not normally expressed in most tissues, but can be induced to do so by several stimuli including growth factors, cytokines, and tumor promoters.[16] Ultraviolet radiation is a known stimulus for COX2 expression in the epidermis.[17],[18] Ultraviolet-induced COX2 expression increases prostaglandin E2.[19]
COX2 gene is mapped to chromosome 1q25.2–q25.3 and is about 8.3 kb in size.[20] Polymorphisms may either eliminate or create binding sites for various factors, potentially altering the expression of COX2. Among the several single nucleotide polymorphisms in COX2, promoter polymorphisms COX2-1195A/G and -765G/C have been shown to modify COX2 transcription and messenger ribonucleic acid levels.[21]COX2-765G/C polymorphisms were associated with the production of prostaglandin E2.[22]
Considering the important role of COX2 in ultraviolet-induced prostaglandin E2 production; ethnic variations in the frequency of single nucleotide polymorphisms in the COX2 gene;[23],[24] and the relation between some of these single nucleotide polymorphisms and the occurrence of vitiligo in Chinese population, we aimed to find out if two of the common COX2 gene polymorphisms were associated with a predisposition to vitiligo among Egyptian patients and if they were related to any specific disease subtypes.[25] Study of these polymorphisms among Egyptians has not been previously evaluated.
Patients and Methods
Patients
This study included 100 patients with vitiligo and 100 healthy individuals as controls. All participants were recruited from the dermatology outpatient clinics of Ain Shams University Hospital, Cairo, Egypt and National Research Centre, Giza, Egypt. Vitiligo was classified as segmental or nonsegmental.[26] Segmental vitiligo was diagnosed if the disease had a dermatomal distribution. Active vitiligo was defined as the appearance of new lesions or the enlargement of existing lesions in the three months before presentation.[25]
Age of the patients ranged from 10 to 71 years. Twenty-four patients were males and 76 were females. Twenty-four patients had positive family history of vitiligo. Age of the persons in the control group ranged from 11–73 years. Twenty-six patients were males and 74 were females. In the patients' group, disease duration ranged from 0.5 to 11 years. Ninety-two patients had generalized and 8 had segmental vitiligo. Sixty-nine patients had active and 31 had inactive disease.
All participants gave informed consent to participate in this work. The study was approved by the research ethics committee of National Research Centre, Giza, Egypt.
All participants were subjected to full history taking, general and dermatological examination, and venous blood sample collection for the study of COX2 -765G/C and -1195A/G gene polymorphisms.
Methods
Deoxyribonucleic acid extraction
Two ml venous blood samples were collected in the morning into ethylene diamine tetraacetic acid (EDTA) vacutainer tubes used for genomic deoxyribonucleic acid extraction. Extraction of genomic deoxyribonucleic acid from whole blood using purification of spin column QIA gene extraction kit was carried out.
Genotyping of -765G/C and -1195A/G polymorphisms
Restriction fragment length polymorphism polymerase chain reaction analysis was used to amplify the COX2 polymorphisms (-765G/C and -1195A/G) according to Zhang et al., 2005.[21] The primers used to amplify the target fragments containing these two polymorphisms were: 5′-TCTCACCCTCA-CATGCTCCT-30 (forward) and 5′-TCTTTTCTGTCCACTTTTCCAA-3′ (reverse) for COX2-1195A/G; 5′-ATGAGGAGAATTTACCTTTCGC-3′ (forward) and 50-TTTTGTGGAATGAAATAGCTACCT-3′ (reverse) for COX2-765G/C.
The polymerase chain reaction was performed in a total volume of 25 μL using 10 pmol of each primer, 1.5 mM Hgcl2, 200 μM dNTPs, and 2U of Taq deoxy ribonucleic acid polymerase. The conditions were as follows: 35 cycles, each consisting of denaturation at 94°C for 30 seconds, annealing at 61°C for 30 seconds, and extension at 72°C for 30 seconds. The reaction cycles were preceded by 5 minutes denaturation at 94°C and were followed by 7 minute extension at 72°C.
The polymerase chain reaction products were confirmed by electropheresis on 2% agarose gel and visualized by ethidium bromide staining.
Statistical analysis
Continuous variables were expressed as mean and standard deviation (SD). Categorical variables were expressed as frequencies and percentages. Student's t-test and analysis of variance (ANOVA) test were used to assess the statistical significance of the difference between two and more than two study group means, respectively. Chi square and Fisher's exact test were used to examine the relationship between categorical variables. Logistic regression was used for calculating odds ratio. A significance level of P < 0.05 was used in all tests. All statistical procedures were carried out using statistical package for the social sciences (SPSS) version 15 for Windows (SPSS Inc, Chicago, Illinois, United States of America).
Results
The mean age of patients was 29.27 ± 11.95 years. Twenty four patients (24%) were males and 76 (76%) were females. Twenty-four (24%) of the patients had positive family history of vitiligo. Mean age of participants in the control group was 28.18 ± 10.56 years. Twenty six (26%) among them were males and 74 (74%) were females. No statistically significant difference was found between patients' group and control group regarding age or sex (P = 0.360 and 0.744, respectively).
In the patients' group, the mean disease duration was 2.7 ± 1.78 years. Ninety-two (92%) patients had generalized and 8 (8%) had segmental vitiligo. Sixty-nine (69%) had active and 31 (31%) had inactive disease.
COX2-1195A/G gene characteristics
Twenty seven (27%) among the vitiligo patients had COX2-1195 AA genotype compared to twenty four (24%) among controls (P = 0.626). Fifty seven (57%) had AG genotype compared to fifty (50%) among controls (P = 0.321). Sixteen (16%) had GG genotype compared to 26 (26%) among controls (P = 0.08). [Table - 1] Subgroup analysis of each of generalized vitiligo and segmental vitiligo against controls showed no significant difference regarding COX2-1195 AA, AG, and GG genotype frequencies (P = 0.739, 0.291, and 0.101 for generalized vitiligo vs. controls; 0.410, 1.00, and 0.676 for segmental vitiligo vs. controls, respectively).
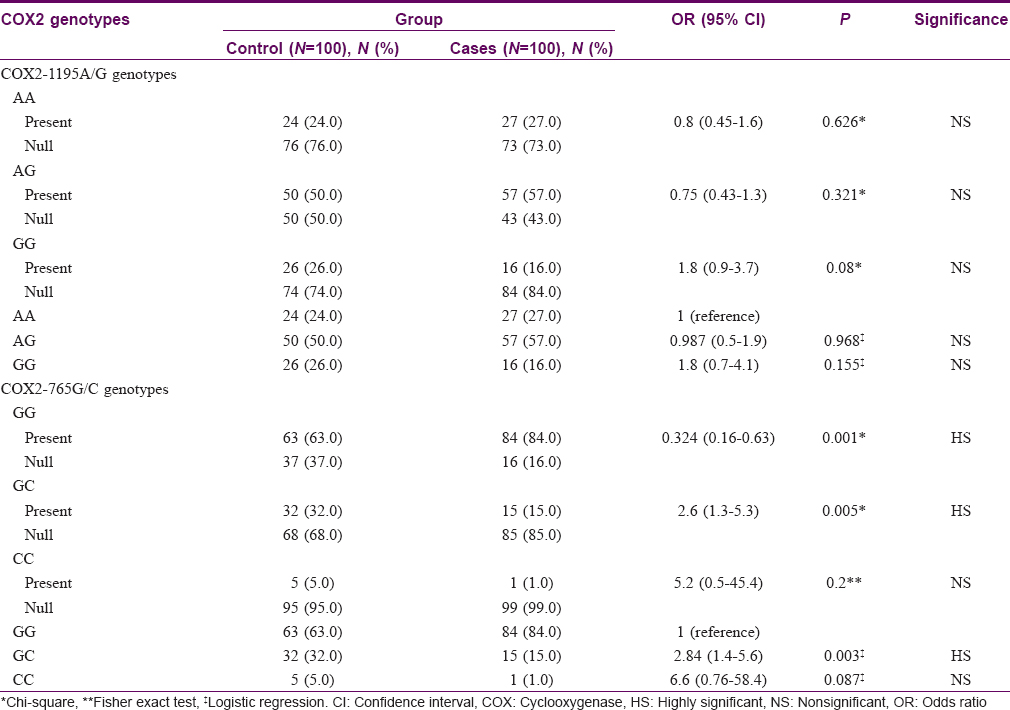
Comparison of vitiligo patients of different COX2-1195A/G genotypes with age and sex showed no significant difference (P = 0.566 and 0.688, respectively). Twelve (44.4%) patients with AA genotype had positive family history of vitiligo, compared to 4 (25%) with GG genotype and 8 (14%) with AG genotype (P = 0.010).
Analyzing COX2-1195 AA, AG, and GG genotype carrier versus non-carrier patients with the different demographic data showed no statistically significant difference with age and sex (P = 0.4460 and 0.423, respectively, for AA genotype; 0.955 and 0.427, respectively, for AG genotype; 0.397 and 1.00, respectively, for GG genotype). However, vitiligo carriers of COX2-1195 AA genotype had a significantly higher percentage of positive family history of the disease [12 (44.4%)] than non-carriers [12 (16.4%)] (P = 0.004), while COX2-1195 AG genotype carriers showed a significantly lower percentage of positive family history of vitiligo [8 (14%)] than non-carriers [16 (37.2%)] (P = 0.007); no significant difference was found among patients with COX2-1195 GG genotype in this regard (P = 1.00). Furthermore, no statistically significant difference was detected regarding the disease duration, subtype and activity on comparing different COX2-1195 genotypes (P = 0.086, 0.874, and 0.682, respectively) or between carriers and non-carriers of each genotype (P = 0.028, 0.443, and 0.427, respectively, for AA genotype; 0.189, 0.722, and 0.770, respectively, for AG genotype; 0.384, 1.00, and 0.770, respectively, for GG genotype).
COX2-765G/C gene characteristics
Eighty four (84%) patients had GG genotype, fifteen (15%) had GC genotype, and one (1%) had CC genotype; whereas among controls, sixty three (63%) had GG genotype, thirty two (32%) had GC genotype, and five (5%) had CC genotype. GG genotype was significantly more frequent (P = 0.001), while GC genotype was significantly less frequent (P = 0.005) among patients than among controls. Frequency of CC genotype showed no significant difference between the groups (P = 0.200). Persons with COX2-765 GC genotype had a 2.6-fold lower risk of having vitiligo [Table - 1].
Seventy seven (83.7%) patients with generalized vitiligo had GG genotype compared to 63 (63%) among controls (P = 0.001). Fourteen (15.2%) patients with generalized vitiligo had GC genotype compared to 32 (32%) among controls (P = 0.006). No significant difference was found between the two groups regarding CC genotype (P = 0.214). COX2-765 GC genotype had a 2.7-fold lower risk of having generalized vitiligo than controls [Table - 2].

Comparison between segmental vitiligo group and controls regarding COX2 -765G/C gene characteristics showed no statistically significant difference (P = 0.256, 0.430, and 1.00 for GG, GC, and CC genotypes, respectively).
Comparison between vitiligo patients with different COX2-765G/C genotypes regarding age, sex, family history of vitiligo (P = 0.309, 0.078, and 0.816, respectively), as well as disease duration, subtype, and activity showed no statistically significant difference between the two groups (P = 0.880, 1.00, and 0.682, respectively).
Comparison between patients carrying COX2-765 GG genotype and CC genotype versus non-carriers showed no statistically significant difference regarding age, sex, or family history of vitiligo (P = 0.780, 0.057, and 1.00, respectively, for GG genotype; 0.125, 1.00, and 1.00, respectively, for CC genotype). Comparison between patients carrying and not carrying COX2-765 GC genotype showed no statistically significant difference regarding age and family history of vitiligo (P = 0.890 and 0.752, respectively), but females were more frequent among patients with COX2-765 GC genotype (P = 0.047).
Comparison between patients with and without COX2-765 GG genotype, GC genotype, and CC genotype regarding disease duration, subtype, and activity showed no statistically significant difference (P = 0.822, 1.00, and 0.770, respectively, for GG genotype; 0.734, 1.00, and 0.772, respectively, for GC genotype; 0.697, 1.00, and 1.00, respectively, for CC genotype).
Discussion
Patients with vitiligo (including its generalized and segmental subtypes) did not show significant difference in the frequency of COX2-1195A/G genotypes when compared with a control group. So, COX2-1195A/G gene polymorphism does not appear to affect the risk of developing vitiligo. Though the over all frequency of different genotypes of COX2-1195A/G was similar in our study population compared to a Chinese population as reported by Li et al., they had reported that the risk of vitiligo was associated with the COX2-1195A/G polymorphism.[25] This difference may be because the effect of these gene polymorphisms may be different among different ethnic populations.
Frequencies of COX2-1195A/G genotypes did not seem to vary with age, sex, disease duration, subtype or activity. However, family history was found to be related with this polymorphism; as patients with -1195 AA genotype showed a significantly higher percentage and patients with AG genotype showed a significantly lower percentage of positive family history of vitiligo. This denotes that COX2-1195A/G gene polymorphism may be related to familial occurrence of the disease.
Regarding COX2-765G/C polymorphism, we found that GG genotype was significantly more frequent whereas GC genotype was significantly less frequent among patients (including those with generalized vitiligo) compared to the control group. Patients with COX2-765 GC genotype have a lower risk of having vitiligo and specifically the generalized type. So COX2-765 GC genotype seems protective against vitiligo. This could be as a result of enhanced prostaglandin biosynthesis as a functional consequence of −765G/C polymorphism.[21] This finding is biologically rational as the −765G/C change also creates a binding element for the E2F transcription factor, a cyclin-dependent regulator of expression of several genes.[21] In contrast to generalized vitiligo, we found that the segmental type is not related to COX2-765G/C gene polymorphism. These results are contradictory to that of Li et al. which found no relationship between the COX2-765 polymorphism and vitiligo risk.[25] It is possible that rare functional alleles are present in linkage disequilibrium with the alleles assessed in the present study.,[21] Our results may explain the beneficial effect of topical prostaglandin E2 in the treatment of localized vitiligo.[11],[12]
Age, sex, family history of vitiligo, disease duration, subtype, and activity of disease did not vary significantly among patients with different COX2-765G/C genotypes. However, a significantly higher frequency of females was noted among patients with COX2 − 765 GC genotype. Considering that patients with COX2-765 GC genotype have a lower risk of having vitiligo, the possibility that females could be less susceptible to vitiligo than males needs further investigation.
Limitation of this work is the relatively small number of participants as this is a pilot study. Studies with a larger sample size are needed to validate our findings.
Conclusion
COX2-1195A/G gene polymorphism was not related to the risk of developing vitiligo or to vitiligo subtypes in the Egyptian population studied. However, COX2-765 GG genotype was associated with a higher susceptibility to develop vitiligo especially of the generalized type. These results may support the possible beneficial effect of topical prostaglandins in vitiligo treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Taïeb A, Picardo M. Clinical practice. Vitiligo. N Engl J Med 2009;360:160-9.
[Google Scholar]
|
| 2. |
Passeron T, Ortonne JP. Physiopathology and genetics of vitiligo. J Autoimmun 2005;25:63-8.
[Google Scholar]
|
| 3. |
Khunger N. Vitiligo surgery. In: Robinson JK, editor. Surgery of the Skin: Procedural Dermatology. 2nd ed. UK: Elsevier Health Sciences; 2010. p. 683-92.
[Google Scholar]
|
| 4. |
Spritz RA. Recent progress in the genetics of generalized vitiligo. J Genet Genomics 2011;38:271-8.
[Google Scholar]
|
| 5. |
Czajkowski R, Mecinska-Jundzill K. Current aspects of vitiligo genetics. Postepy Dermatol Alergol 2014;31:247-55.
[Google Scholar]
|
| 6. |
Parsad D, Pandhi R, Dogra S, Kumar B. Topical prostaglandin analog (PGE2) in vitiligo – A preliminary study. Int J Dermatol 2002;41:942-5.
[Google Scholar]
|
| 7. |
Sakai Y, Fujita K, Sakai H, Mizuno K. Prostaglandin E2 regulates the expression of basic fibroblast growth factor messenger RNA in normal human fibroblasts. Kobe J Med Sci 2001;47:35-45.
[Google Scholar]
|
| 8. |
Puri N, van der Weel MB, de Wit FS, Asghar SS, Das PK, Ramaiah A, et al. Basic fibroblast growth factor promotes melanin synthesis by melanocytes. Arch Dermatol Res 1996;288:633-5.
[Google Scholar]
|
| 9. |
Hempel SL, Wessels DA. Prostaglandin E2 synthesis after oxidant stress is dependent on cell glutathione content. Am J Physiol 1994;266(5 Pt 1):C1392-9.
[Google Scholar]
|
| 10. |
Elmets CA, Ledet JJ, Athar M. Cyclooxygenases: Mediators of UV-induced skin cancer and potential targets for prevention. J Invest Dermatol 2014;134:2497-502.
[Google Scholar]
|
| 11. |
Kapoor R, Phiske MM, Jerajani HR. Evaluation of safety and efficacy of topical prostaglandin E2 in treatment of vitiligo. Br J Dermatol 2009;160:861-3.
[Google Scholar]
|
| 12. |
Anbar TS, El-Ammawi TS, Abdel-Rahman AT, Hanna MR. The effect of latanoprost on vitiligo: A preliminary comparative study. Int J Dermatol 2015;54:587-93.
[Google Scholar]
|
| 13. |
DeWitt DL. Prostaglandin endoperoxide synthase: Regulation of enzyme expression. Biochim Biophys Acta 1991;1083:121-34.
[Google Scholar]
|
| 14. |
Gharib AF, Karam RA, Abd El Rahman TM, Elsawy WH. COX-2 polymorphisms -765G→C and -1195A→G and hepatocellular carcinoma risk. Gene 2014;543:234-6.
[Google Scholar]
|
| 15. |
Mohamed FZ, Hussein YM, El-Deen IM, Sabea MS. Cyclooxygenase-2 single-nucleotide polymorphisms and hepatocellular carcinoma in Egypt. Mol Biol Rep 2014;41:1461-8.
[Google Scholar]
|
| 16. |
Rundhaug JE, Fischer SM. Cyclo-oxygenase-2 plays a critical role in UV-induced skin carcinogenesis. Photochem Photobiol 2008;84:322-9.
[Google Scholar]
|
| 17. |
An KP, Athar M, Tang X, Katiyar SK, Russo J, Beech J, et al. Cyclooxygenase-2 expression in murine and human nonmelanoma skin cancers: Implications for therapeutic approaches. Photochem Photobiol 2002;76:73-80.
[Google Scholar]
|
| 18. |
Rodriguez-Burford C, Tu JH, Mercurio M, Carey D, Han R, Gordon G, et al. Selective cyclooxygenase-2 inhibition produces heterogeneous erythema response to ultraviolet irradiation. J Invest Dermatol 2005;125:1317-20.
[Google Scholar]
|
| 19. |
Rundhaug JE, Simper MS, Surh I, Fischer SM. The role of the EP receptors for prostaglandin E2 in skin and skin cancer. Cancer Metastasis Rev 2011;30:465-80.
[Google Scholar]
|
| 20. |
Tay A, Squire JA, Goldberg H, Skorecki K. Assignment of the human prostaglandin-endoperoxide synthase 2 (PTGS2) gene to 1q25 by fluorescence in situ hybridization. Genomics 1994;23:718-9.
[Google Scholar]
|
| 21. |
Zhang X, Miao X, Tan W, Ning B, Liu Z, Hong Y, et al. Identification of functional genetic variants in cyclooxygenase-2 and their association with risk of esophageal cancer. Gastroenterology 2005;129:565-76.
[Google Scholar]
|
| 22. |
Sanak M, Szczeklik W, Szczeklik A. Association of COX-2 gene haplotypes with prostaglandins production in bronchial asthma. J Allergy Clin Immunol 2005;116:221-3.
[Google Scholar]
|
| 23. |
Cox DG, Pontes C, Guino E, Navarro M, Osorio A, Canzian F, et al. Polymorphisms in prostaglandin synthase 2/cyclooxygenase 2 (PTGS2/COX2) and risk of colorectal cancer. Br J Cancer 2004;91:339-43.
[Google Scholar]
|
| 24. |
Panguluri RC, Long LO, Chen W, Wang S, Coulibaly A, Ukoli F, et al. COX-2 gene promoter haplotypes and prostate cancer risk. Carcinogenesis 2004;25:961-6.
[Google Scholar]
|
| 25. |
Li M, Gao Y, Li C, Liu L, Li K, Gao L, et al. Association of COX2 functional polymorphisms and the risk of vitiligo in Chinese populations. J Dermatol Sci 2009;53:176-81.
[Google Scholar]
|
| 26. |
Dave S, Thappa DM, Dsouza M. Clinical predictors of outcome in vitiligo. Indian J Dermatol Venereol Leprol 2002;68:323-5.
[Google Scholar]
|
Fulltext Views
2,058
PDF downloads
784





