Translate this page into:
Reduced immunohistochemical expression of CCN3 in vitiligo
2 Department of Pathology, Hospital ErastoGaertner, Curitiba, Paraná, Brazil
3 Department of Dermatology, School of Medicine, Pontifical Catholic University of Paraná, Santa Casa De Misericórdia Hospital, Curitiba, Paraná, Brazil
4 Department of Pathology, Graduate Program in Health Sciences, School of Medicine, Pontifical Catholic University of Paraná, Curitiba, Paraná, Brazil
Correspondence Address:
Adriane Reichert Faria
Rua Padre Anchieta, 1846, Sala 1003, CEP 80.730-000, Curitiba, Paraná
Brazil
| How to cite this article: Faria AR, Jung JE, Silva de Catro CC, de Noronha L. Reduced immunohistochemical expression of CCN3 in vitiligo. Indian J Dermatol Venereol Leprol 2018;84:558-562 |
Abstract
Background: Defective adhesion seems to be involved in the chronic loss of melanocytes observed in vitiligo. Recent findings showed an association of genetic variants of an adhesion gene with vitiligo and reduced immunohistochemical expression of some adhesion molecules in vitiligo skin.
Aims: To compare CCN3 immunohistochemical expression in lesional and non-lesional epidermis of individuals with vitiligo.
Methods: A total of 66 skin specimens from 33 volunteers with vitiligo were analyzed by immunohistochemistry using anti-CCN3 antibodies. Absence of topical or systemic treatment for vitiligo over the previous 30 days and availability of an area of non-lesional skin for biopsy at least 15 cm away from any vitiliginous macules were the main inclusion criteria.
Results: A significant reduction of CCN3 expression was observed in lesional skin as compared to non-lesional skin (P = 0.001).
Limitations: Paraffin embedded skin samples do not allow investigation by molecular biology methods. Not all samples allowed analysis due to the lamina preparation technique. Complete clinical data was not available for all patients.
Conclusion: Our results support the hypothesis of impaired cell adhesion in vitiligo suggested by genetic studies. The pattern of immunohistochemical expression suggests that vitiligo might be an epithelial disease and not just a melanocyte disorder.
Introduction
Vitiligo vulgaris is an acquired depigmenting disorder of unknown etiology characterized by white, often symmetrical skin patches caused by a loss of functioning epidermal and sometimes hair follicle melanocytes.[1],[2] Among several theories to explain the disease, one suggests that defective cell adhesion is involved in the chronic loss of melanocytes observed in vitiligo.[3],[4]
Genetic variants of the DDR1(discoidin domain receptor 1) gene have been associated with vitiligo, and DDR1 immunohistochemical expression in vitiligo skin was found significantly reduced in comparison with non-lesional skin, findings compatible with the adhesion hypothesis of vitiligo pathogenesis.[5],[6],[7]
DDR1 is a tyrosine kinase receptor that was found responsible for the adhesive properties of CCN3, a matricellular protein required for the 3D organization of the melanocyte network on the basal membrane of human skin equivalent that was also shown to inhibit the proliferation of melanocytes and stimulate the adhesion of melanocytes to the basal membrane collagen IV.[8]
Data linking CCN3 to vitiligo is scanty. Here, we present histopathological data showing reduced CCN3 expression in vitiligo skin.
Methods
The study was approved by the research Ethics Committee of Pontifical Catholic University of Paraná and was conducted at the Santa Casa de Misericórdia de Curitiba Hospital according the principles of the Declaration of Helsinki.
A total of 33 patients evaluated by a single dermatologist (CCSC) were enrolled. The main inclusion criteria were: absence of topical or systemic treatment for vitiligo over the previous 30 days and availability of an area of non-lesional skin for biopsy within at least 15 cm away from any vitiligo macules, since skin biopsies up to 15 cm have already shown alterations not found in non-lesional skin.[9]
After obtaining signed informed consent, two skin specimens from each volunteer (vitiligo lesional and non-lesional skin) were obtained with a 3-mm punch. The vitiligo specimen was obtained from a well-defined achromic area, without any clinical sign of inflammation. Formalin-fixed paraffin-embedded skin samples were prepared using the tissue microarray technique. Immunohistochemistry was performed with CCN3 antibody (Abcam) at a dilution of 1:600 μl.
Out of the original 33, 30 pairs of samples were found adequate for microscopic analysis – samples from the other three patients were not included due to inappropriate reaction. All the epidermal fields analyzed were first separated with the Photoshop program, to ensure that corneal and dermal deposition of CCN3 did not influence the results, allowing the epidermal area to be correctly calculated.
One good quality section with immunohistochemical staining for anti-CCN3 was chosen to serve as a “mask”: in this field, the observer manually selected the degrees of brown coloration to be considered positive for CCN3 expression, establishing a pattern to be followed in the analysis [Figure - 1]. From within the area of immunohistochemical positivity in each skin specimen (×200), three fields underwent the morphometric analysis, aiming to compare the expression of CCN3 between vitiligo and nonlesional skin [Figure - 1] and [Figure - 2]. Analysis was performed using the Image Pro Plus® software.
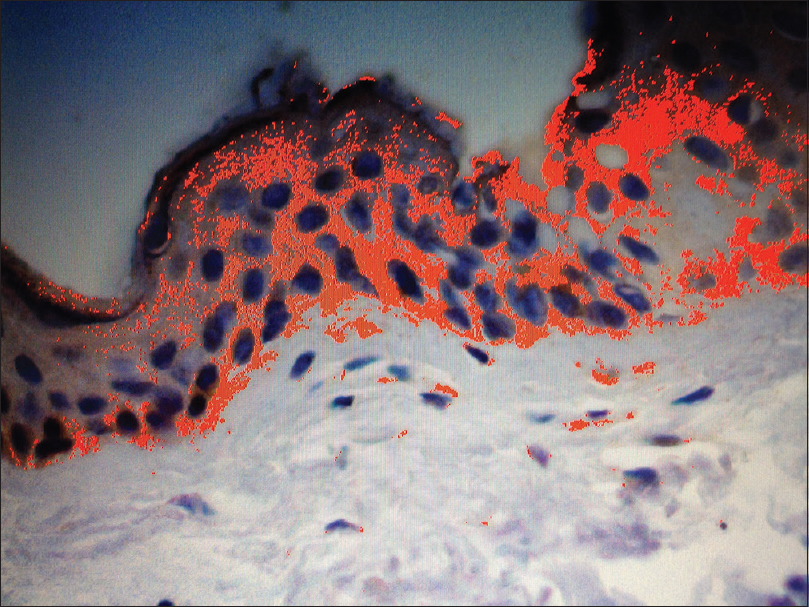 |
| Figure 1: Non-lesional skin from vitiligo patients. Image Pro Plus™ analysis of non-lesional skin, showing positive immunohistochemical reaction in red, the total area being expressed in μm2. Positive immunohistochemical reaction has been identified by the software through a sample of brown coloration considered appropriate by the observer, with the pattern being followed for all fields analysed. The sum of the areas with positive epidermal reaction represented the immunohistochemical expression of CCN3 for high-power field in μm2 (anti-CCN3, ×200) |
 |
| Figure 2: Vitiligo skin. Image Pro Plus™ analysis of vitiligo skin, shows the positive immunohistochemical reaction in red, the total area being expressed in μm2. Positive immunohistochemical reaction has been identified by the software through a sample of brown coloration considered appropriate by the observer, with the pattern being followed for all fields analyzed. The sum of the areas with positive epidermal reaction represented the immunohistochemical expression of CCN3 for high-power field in μm2 (anti-CCN3, ×200) |
In order to exclude any influence due to skin thickness, each field had its total epidermal area calculated; for each specimen evaluated, the percentage of CCN3 per area was used for the comparison.
Differences in the expression of CCN3 were analyzed using the non-parametric Wilcoxon test for quantitative variables as implemented in the Statistica software v. 8.0 [StatSoft, Palo Alto, USA]. A P value < 0.05 indicated statistical significance.
Results
Clinical characteristics and biopsy sites of all patients studied are detailed in [Table - 1].

The cellular pattern of positive immunohistochemical reaction observed for anti-CCN3 antibody was predominantly cytoplasmic, mainly on keratinocytes and melanocytes. The tissue pattern of staining was predominantly basal and parabasal, areas near collagen IV.
Results of the comparative analysis of CCN3 expression between vitiligo and non-lesional skin in 30 patients are presented in [Table - 2]. The mean area of immunohistochemical positivity for CCN3 in vitiligo samples was lower than in nonlesional skin (11181 μm[2] vs. 24397 μm[2] respectively; P = 0.001). A comparison of the percentage area of CCN3 positivity in vitiligo and non-lesional skin also showed a reduced expression in vitiligo. (2.91 vs. 7.04 respectively; P = 0.001).

There were no correlations between the total area of immunopositive reaction or percentage of immunopositivity with disease evolution period or DDR1 expression in vitiligo and non-lesional samples.
Discussion
Vitiligo is an acquired depigmenting disorder characterized by the loss of functioning epidermal melanocytes probably by multiple and overlapping pathogenic mechanisms.[10]
Lesional skin of vitiligo patients lacks melanocytes.[11] It has been proposed that defective adhesion of melanocytes is a predisposing factor, and mechanical trauma, the main disease trigger. This phenomenon, named melanocytorrhagy, is the basis of a theory that considers vitiligo to be caused by the chronic detachment and transepidermal loss of melanocytes.[3] According to this hypothesis,in vivo friction of perilesional non-segmental vitiligo skin induces the detachment of living melanocytes from the basal layer, followed by transepidermal migration and eventual death of the detached pigment cells.[4],[12]
Differentiated human melanocytes remain strictly localized at the basement membrane and cannot survive within the upper epidermal layers unless transformed as in nevi or melanoma.[8] Melanocytes can occasionally change their characteristicsin vivo when they become migratory and proliferative during re-epithelialization of wounds and repigmentation in non-segmental vitiligo.[13] Recently, it has been demonstrated that melanocytes from unstable vitiligo show significantly lower adhesion to collagen type IV than melanocytes in controls and in stable vitiligo.[4]
In the epidermis, attachment of melanocytes to collagen IV is mediated through DDR1, which is under the control of CCN3.[14] CCN3 belongs to a group of structurally related intracellular proteins which are also secreted and bound to the extracellular matrix, indicating an important role for members of this family in internal and external cell-signaling pathways. CCN functions seem to be predominantly mediated via extracellular cell adhesion receptors such as integrins.[15]
The CCN family consists of six members, cysteine-rich 61 (CYR61, CCN1), connective tissue growth factor (CTGF, CCN2), nephroblastoma overexpressed (NOV, CCN3), and Wnt-induced secreted proteins (WISP1-3, CCN4-6), which are involved in the regulation of various cellular processes such as proliferation, adhesion, migration and differentiation.[16]
Fukunaga-Kalabis et al. have demonstrated that keratinocytes in culture or human skin did not express CCN3, whereas melanocytes constitutively expressed it at low levels; melanocytes overexpressing CCN3 were localized to the basement membrane zone of human skin reconstructs. Knockdown of CCN3 in melanocytes increased their numbers and changed their localization either upward to the suprabasal layers of the epidermis or downwards into the dermis.[8]
The present study shows a decrease of CCN3 expression in vitiligo skin. Sampling in all 30 cases was standardized requiring clinically depigmented skin for lesional biopsies and normal non-lesional area selected for biopsy at least 15 cm away from any achromic patch. Ricard et al. found higher CCN3 expression in keratinocytes of lesional skin than in normal control in 9 out of 12 patients, in a semi-quantitative assessment of CCN3 by immunofluorescence. However they had a heterogeneous group (8 patients' biopsies were taken from non-lesional and vitiligo areas, 3 patients' from perilesional and vitiligo areas, and 1 patient's biopsy was performed at the perilesional-lesional junction).[14]
We also observed CCN3 protein expression in a cytoplasmic pattern in keratinocytes and melanocytes, as already described.[14]
Although in vivo CCN3 results are antagonistic, we also have found reduced expression of DDR1 in vitiligo compared to non-vitiligo skin.[7],[14]
In vitro results of the same authors confirm that CCN3 mediates adhesion of melanocytes to collagen IV and that inhibition of CCN3 induces the detachment of melanocytes.[14] Studies involving CCN3 silencing in melanocytes in epidermal reconstructs showed that basal melanocytes still expressed CCN3, whereas the few detached melanocytes found in all sections did not, suggesting that efficiently silenced melanocytes did not attach during the adhesion period of reconstruction.[14]
Our in vivo findings show a significant reduction of CCN3 in achromic areas, while normal control skin exhibits basal and parabasal expression of CCN3. Our results are in accordance with other studies on normal human skin which demonstrated CCN3 expression at the basal layer of the epidermis, where melanocytes are positioned.[8]
Ricard et al. also showed that CCN3 exerts a regulating effect on DDR1 expression. This paper showed (using in vitro tests) a significant inhibition of melanocyte adhesion to collagen IV when DDR1is silenced, suggesting that DDR1 is probably not the only molecule regulated by CCN3 that is involved with melanocyte adhesion.[14]
A limitation of the present study is that paraffin-embedded skin samples do not allow investigations involving molecular biologic methods. Not all samples allowed analysis due to the lamina preparation technique and complete clinical data was not available for all patients.
CCN3 and CCN5 proteins are prominent in epidermal keratinocytes and are linked to keratinocyte differentiation.[17] Keratinocytes control the normal growth of melanocytes and maintain a lifelong stable ratio; they regulate the expression of cell surface molecules, and as shown by Fukunaga–Kalabis, CCN3 production by melanocytes after their contact with keratinocytes upregulates the DDR1 adhesion receptor for collagen IV and influences melanocyte localization, contributing to homeostasis of the skin.[8]
Vital interactions between normal human skin cells such as melanocytes and keratinocytes are necessary for proper tissue development and maintenance. We consider vitiligo to be a disease not only of melanocytes, but of the epidermis itself. Further investigation is necessary to elucidate how lower CCN3 expression in vitiligo skin might influence the expression of other molecules in keratinocytes and lead to impaired epidermal adhesion.
Acknowledgement
We would like to thank Marina Viola de Azevedo for preparing the biopsy materials.
Financial support and sponsorship
Laboratory of Experimental Pathology of School of Medicine, Pontifical Catholic University of Paraná, Curitiba, Paraná, Brazil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Alikhan A, Felsten LM, Daly M, Petronic-Rosic V. Vitiligo: A comprehensive overview Part I. Introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am AcadDermatol 2011;65:473-91.
[Google Scholar]
|
| 2. |
Taïeb A, Picardo M, VETF Members. The definition and assessment of vitiligo: A consensus report of the Vitiligo European Task Force. Pigment Cell Res 2007;20:27-35.
[Google Scholar]
|
| 3. |
Cario-André M, Pain C, Gauthier Y, Taïeb A. The melanocytorrhagic hypothesis of vitiligo tested on pigmented, stressed, reconstructed epidermis. Pigment Cell Res 2007;20:385-93.
[Google Scholar]
|
| 4. |
Kumar R, Parsad D, Kanwar AJ. Role of apoptosis and melanocytorrhagy: A comparative study of melanocyte adhesion in stable and unstable vitiligo. Br J Dermatol 2011;164:187-91.
[Google Scholar]
|
| 5. |
Liang Y, Yang S, Zhou Y, Gui J, Ren Y, Chen J, et al. Evidence for two susceptibility loci on chromosomes 22q12 and 6p21-p22 in Chinese generalized vitiligo families. J Invest Dermatol 2007;127:2552-7.
[Google Scholar]
|
| 6. |
Silva de Castro CC, do Nascimento LM, Walker G, Werneck RI, Nogoceke E, Mira MT, et al. Genetic variants of the DDR1 gene are associated with vitiligo in two independent Brazilian population samples. J Invest Dermatol 2010;130:1813-8.
[Google Scholar]
|
| 7. |
Reichert-Faria A, Jung JE, Moreschi Neto V, de Castro CC, Mira MT, Noronha L, et al. Reduced immunohistochemical expression of Discoidin Domain Receptor 1 (DDR1) in vitiligo skin. J EurAcadDermatolVenereol 2013;27:1057-9.
[Google Scholar]
|
| 8. |
Fukunaga-Kalabis M, Martinez G, Liu ZJ, Kalabis J, Mrass P, Weninger W, et al. CCN3 controls 3D spatial localization of melanocytes in the human skin through DDR1. J Cell Biol 2006;175:563-9.
[Google Scholar]
|
| 9. |
Moellmann G, Klein-Angerer S, Scollay DA, Nordlund JJ, Lerner AB. Extracellular granular material and degeneration of keratinocytes in the normally pigmented epidermis of patients with vitiligo. J Invest Dermatol 1982;79:321-30.
[Google Scholar]
|
| 10. |
Dell'anna ML, Picardo M. A review and a new hypothesis for non-immunological pathogenetic mechanisms in vitiligo. Pigment Cell Res 2006;19:406-11.
[Google Scholar]
|
| 11. |
Halder RM, Chappell JL. Vitiligo update. SeminCutan Med Surg 2009;28:86-92.
[Google Scholar]
|
| 12. |
Gauthier Y, Cario-Andre M, Lepreux S, Pain C, Taïeb A. Melanocyte detachment after skin friction in non lesional skin of patients with generalized vitiligo. Br J Dermatol 2003;148:95-101.
[Google Scholar]
|
| 13. |
Gauthier Y, Cario Andre M, Taïeb A. A critical appraisal of vitiligo etiologic theories. Is melanocyte loss a melanocytorrhagy? Pigment Cell Res 2003;16:322-32.
[Google Scholar]
|
| 14. |
Ricard AS, Pain C, Daubos A, Ezzedine K, Lamrissi-Garcia I, Bibeyran A, et al. Study of CCN3 (NOV) and DDR1 in normal melanocytes and vitiligo skin. ExpDermatol 2012;21:411-6.
[Google Scholar]
|
| 15. |
Chen CC, Lau LF. Functions and mechanisms of action of CCN3 matricellular proteins. Int J Biochem Cell Biol 2008;41:771-83.
[Google Scholar]
|
| 16. |
Perbal B. CCN proteins: Multifunctional signalling regulators. Lancet 2004;363:62-4.
[Google Scholar]
|
| 17. |
Rittie L, Perbal B, Castellot JJ Jr., Orringer JS, Voorhees JJ, Fisher GJ. Spatial modulation of CCN3 proteins during wound healing in human skin in vivo. J Cell Commun Signal 2011;5:69-80.
[Google Scholar]
|
Fulltext Views
1,725
PDF downloads
1,059





