Translate this page into:
Hair manifestations of endocrine diseases: A brief review
Correspondence Address:
Sunil Dogra
Department of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education and Research, Sector 12, Chandigarh - 160 012
India
| How to cite this article: Vinay K, Sawatkar GU, Dogra S. Hair manifestations of endocrine diseases: A brief review. Indian J Dermatol Venereol Leprol 2018;84:528-538 |
Abstract
Hair disorders are common in clinical practice and depending upon social and ethnic norms, it can cause significant psychosocial distress. Hair growth, cycling and density are regulated by many endogenous factors, mainly circulating hormones. Thus, diseases affecting the endocrine system can cause varied changes in physiological hair growth and cycling. Diagnosis and treatment of these disorders require a multidisciplinary approach involving a dermatologist, gynecologist and an endocrinologist. In this review, we briefly discuss the influence of hormones on the hair cycle and hair changes in various endocrine disorders.
Introduction
Hair is an important structure of the body playing a significant role in the psychosocial personality of an individual. However, endocrine dysfunction can affect the hair growth, cycling and density and its examination can give an insight to the underlying cause. Hormonal disturbances can cause hypertrichosis, hirsutism or alopecia. In this review, we briefly discuss the influence of hormones on the hair cycle and hair changes in various endocrine disorders.
Hormonal Influence on Hair
Androgens
In the development of secondary sexual characters
At puberty, the vellus follicles in the axillae and groin in both males and females, and of the beard area and trunk of males are converted to terminal hair by the action of androgens.[1] At certain sites, androgens increase the duration of hair cycle, alter the ability of keratinocytes to divide, increase pigmentation and increase the size of the dermal papilla.
In patterned hair loss
Circulating androgens play an important role in the pathogenesis and progression of patterned hair loss. This is based on the observation that eunuchs and castrated boys do not develop male patterned hair loss, unless supplemented by testosterone.[2] Also genetically males with androgen insensitivity syndrome do not go bald. In male patterned hair loss, di-hydro testosterone and testosterone bind to androgen receptors present on the cells of dermal papilla and alter the production of soluble regulatory factors that influence the growth and activity of other cells, such as hair follicle keratinocytes. This leads to progressive miniaturization of scalp hairs.[3]
The role of androgens in female patterned hair loss is controversial. Not all studies have found raised androgens in patients with female patterned hair loss. In a study comprising 109 women with female patterned hair loss, 38.5% were found to have a clinical or biochemical evidence of hyperandrogenism.[4] Of the 187 women with hair loss, 67% of those with hair loss alone and 84% who were also hirsute, were found to have some degree of biochemical androgen excess.[5] In patients with normal androgen levels, it is hypothesized that there is increased formation of potent androgens (di-hydro testosterone and testosterone) from their precursors in the skin (cutaneous hyperandrogenism).[6]
Oestrogen
Oestrogens are required for the normal development of pubic and axillary hair in females. However, its function in scalp hair growth in both sexes is less clear as is its role in female patterned hair loss. The increased prevalence of female patterned hair loss after menopause suggests a stimulatory role for oestrogens in hair growth. High systemic oestrogen levels in pregnancy are speculated to partially account for the prolongation of anagen, whereas post-partum fall in oestrogen levels may partially account for telogen gravidarum.[7] On the other hand, in murine models, the administration of parenteral and topical oestrogen agonists has been shown to produce a profound and prolonged inhibition of hair growth through telogen arrest, whereas oestrogen antagonists stimulated hair growth through the initiation of anagen.[8] Thus, unlike androgens, the role of oestrogen on hair cycling is controversial and has not been adequately studied.
Growth hormone
Growth hormone potentiates the effect of androgen on sexual hair growth. About 5-fold more testosterone is required to induce axillary hair in growth hormone-deficient than in growth hormone-sufficient hypogonadal boys.[1] Its effectsare probably mediated through insulin like growth factor-1.
Insulin
Insulin and the insulin like growth factorsystem have a role in stimulating hair growth and may act in concert with the androgens. Hyperinsulinaemia may also induce 5α reductase activity and cause increased production of di-hydro testosterone.
Prolactin
Prolactin induces catagen in male occipital scalp hair follicles,[9] while promoting hair shaft elongation in female fronto-temporal scalp hair follicles.[10] Clinically, prolactin excess is associated with hirsutism, probably because of stimulation of hyperandrogenism.
Thyroid
Thyroid receptors have been found on outer root sheath cells and it appears to regulate the frequency of the hair cycle.[11] Hypothyroidism leads to decreased frequency of anagen, whereas hyperthyroidism leads to thin hairs.
Vitamin D-retinoid X receptor system
Vitamin D is necessary for postnatal cycling of the hair follicle.[12] Individuals born with vitamin D receptor deletions suffer from postnatal alopecia that cannot be corrected by calcium administration.
Definitions[13]
Hirsutism: Presence of excessive terminal hair in androgen-dependent areas of the female body. [Figure - 1]
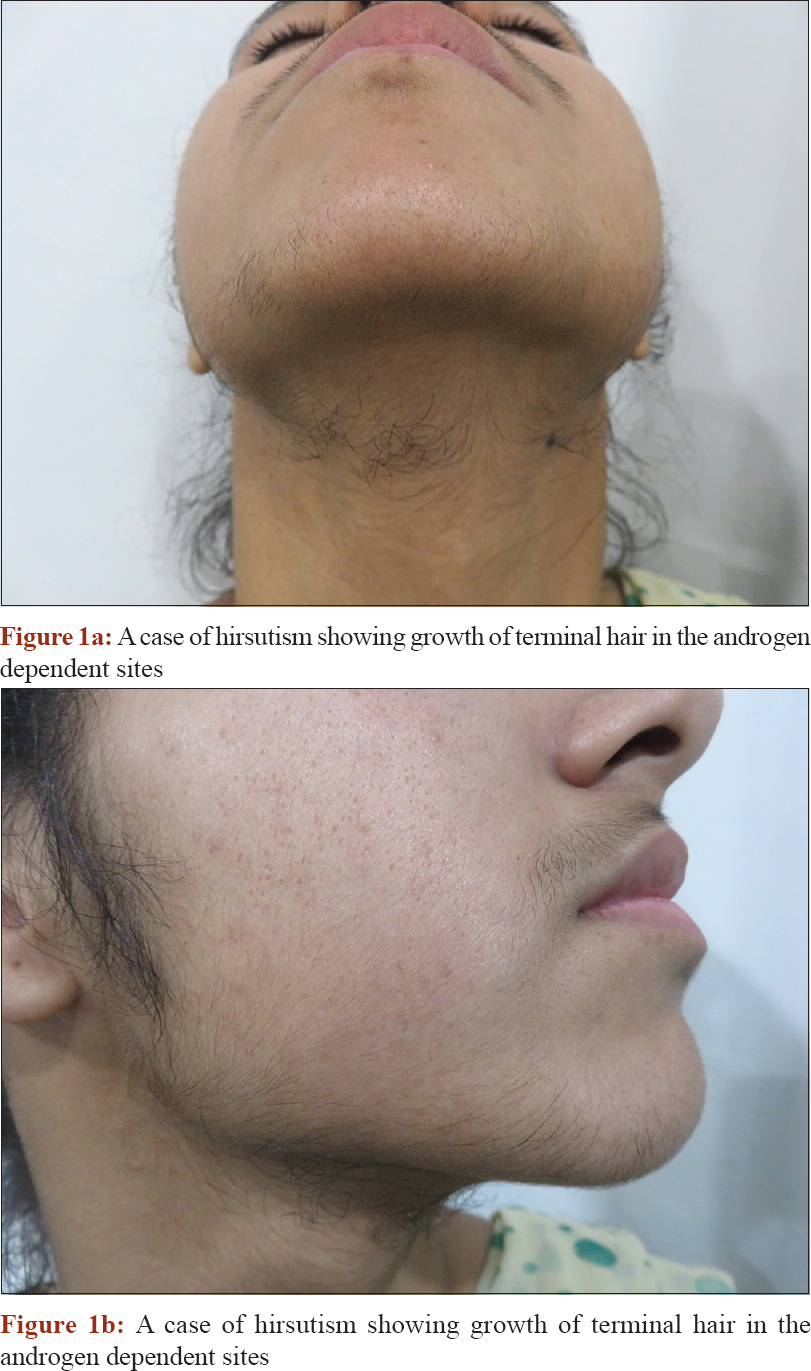 |
| Figure 1: |
Hyperandrogenemia: Abnormally high amounts of androgens detectable in the blood.
Hyperandrogenism: Clinical manifestation of hyperandrogenemia in the form of hirsutism, acne, seborrhea, alopecia, infertility etc.
Cutaneous hyperandrogenism: Cutaneous clinical signs of hyperandrogenism present without documented evidence of hyperandrogenemia [Figure - 2].
 |
| Figure 2: A case of polycystic ovarian syndrome with hirsutism, seborrhea and hormonal acne |
Hypertrichosis: Presence of excessive body hair in non-androgen dependent manner [Figure - 3].
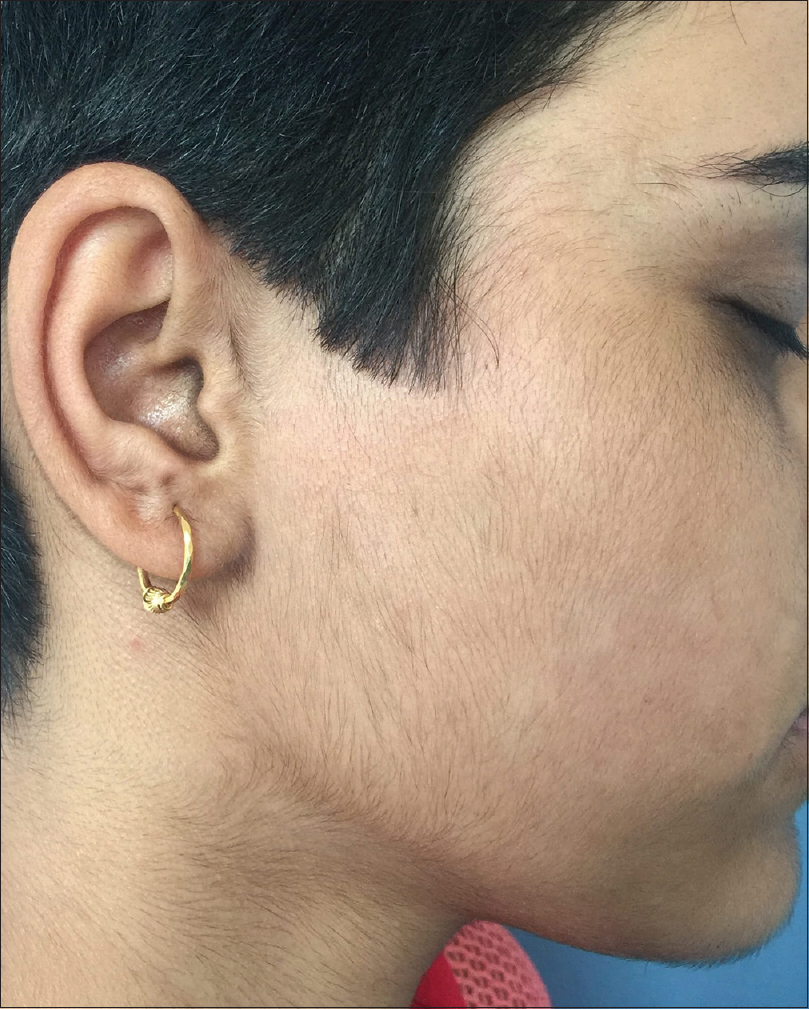 |
| Figure 3: Hypertrichosis secondary to use of systemic cyclosporine and corticosteroids for treatment of alopecia areata |
Endocrine Disorders Causing Abnormal/excessive Hair Growth
Hirsutism
Hirsutism, a clinical sign for hyperandrogenemia, is a frequent reason of cosmetic embarrassment, poor self-esteem, and psychological distress for women world over.[14]
Epidemiology
The prevalence of hirsutism ranges from 4.3 to 10.8% in blacks and whites, but appears to be somewhat lower in Asians.[15] The prevalence and severity of hirsutism decrease with increasing age (except among post-menopausal women).[16]
Causes
Hirsutism is a manifestation of increased action of androgens at the hair follicle. Hirsutism is observed in 70–80% of patients with hyperandrogenemia and in turn, 70–80% of hirsute patients have hyperandrogenemia.[16] The most common cause of hirsutism is polycystic ovarian syndrome, accounting for >70% of cases.[17] Depending on ethnicity and the geographic area, idiopathic hirsutism constitutes 5–17% of the patients with hirsutism. In approximately 1–8% of the women with hirsutism, the underlying cause is non-classical congenital adrenal hyperplasia because of 21-hydroxylase deficiency.[18] Other causes are summarized in [Figure - 4].[17]
 |
| Figure 4: Causes of hirsutism |
Pathophysiology
Vellus hair is converted to terminal hair by the action of androgens at puberty. Increase level of circulating androgens in hyperandrogenemic states stimulates the differentiation of androgen dependent vellus hair into terminal hair. Various other mechanisms postulated in idiopathic hirsutism are[6],[11]
- Exaggerated peripheral 5α reductaseactivity
- Androgen receptor polymorphism
- Altered androgen metabolism
- Insulin resistance
- Decreased sex hormone binding globulin levels
- Lower aromatase levels.
Approach
A detailed history regarding the age at onset, duration, menstrual irregularities, weight gain, diabetes, symptoms of hyperandrogenemia (acne, seborrhea, alopecia etc.), symptoms of virilization (deepening of voice, increased muscularity, breast atrophy etc.), drug intake, galactorrhoea, family history etc., should be obtained. Functional causes usually show a peripubertal onset, slow progression, positive family history in some and signs of virilization are extremely rare. On the other hand, androgen secreting tumors show sudden onset, rapid progression, signs and symptoms of virilization in middle aged or elderly females.[15] A detailed physical examination of the patient should be done. All the androgen dependent areas should be assessed for the severity of hirsutism. Truncal hirsutism is a better indicator of polycystic ovarian syndrome than facial hirsutism.[19] Signs of virilization such as clitoromegaly or thinning of scalp hair should be checked for. Abdomen should be palpated for a mass. Blood pressure of the patient should be recorded and body mass index calculated.
Severity assessment and investigations
The subjective and objective assessment of hirsutism is summarized in [Table - 1].[20]

The relevant investigations are tabulated in [Table - 2].[21],[22],[23]

Treatment
Management of clinically important hirsutism consists of a dual approach: medical therapy of the underlying abnormalityand physical methods to remove the terminal hairs already present.[24],[25] The medical and physical methods are summarized in [Table - 3] and [Table - 4] respectively.


Summary of medical treatment
- Oral contraceptive pillsprovide effective contraception recommended for the concomitant use of anti-androgens, and are quite useful for the regularization of menstrual bleeding in women with polycystic ovarian syndrome, which will also reduce the risk of endometrial hyperplasia.
- Oral contraceptive pills/anti-androgen combinations are more efficacious than oral contraceptive pills monotherapy[26] Oral contraceptive pillsare usually combined in majority of women and anti-androgen monotherapy should be used only when strict contraception is ensured[27]
- Evidence suggests a potency gradient with anti-androgenic oral contraceptive pillss cyproterone acetate >drospirenone >chlormadinone acetate and third-generation oral contraceptive pills being superior to second- generation oral contraceptive pills for hirsutism (desogestrel >levonorgestrel)[25]
- Among anti-androgens, flutamide appears to have highest efficacy and finasteride the lowest[15]
- Metformin has beneficial effects in overweight or obese patients and is especially helpful in adolescents with polycystic ovarian syndrome[28]
- In women with congenital adrenal hyperplasia, prolonged remission after withdrawal of anti-androgen therapy may be obtained by the addition of glucocorticoids
- For all pharmacological therapies, a trial of 6 months is suggested before any changes in the medication can be made[27]
- For women undergoing photoepilation, addition of eflornithine cream (13.9%) is suggested for a more rapid response.[27]
Hypertrichosis [Figure - 3]
The causes of hypertrichosis are varied and can be congenital and acquired. Unlike hirsutism, where endocrine abnormalities are the predominant cause, only a minority of cases of acquired hypertrichosis has an endocrine cause. The important endocrine causes of acquired hypertrichosis are Cushing's syndrome [Figure - 3], acromegaly and hypothyroidism.[29] The exact mechanism by which endocrine disturbances cause hypertrichosis is not known, but their effect on hair growth and cycling may have a role. The speculated mechanisms are (a) switching of vellus to terminal hairs or (b) prolongation of the anagen duration.[30]
The treatment is mainly directed at treatment of the underlying endocrine dysfunction. Significant cosmetic improvement can be obtained by physical treatment modalities [Table - 4], which are usually combined with medical therapy.
Endocrine Disorders Causing or Associated With Hair Loss
Patterned hair loss
Sex hormones play an important role in the development of patterned hair loss in both males and females. male patterned hair losswas thought to occur in men with normal androgen levels and is predominantly caused by genetic polymorphism of androgen receptor. However, recent studies evaluating the hormonal profile of early androgenetic alopecia in men have found hormonal disturbances akin to polycystic ovarian syndrome.[31]
Female patterned hair loss
Female patterned hair loss is characterized by progressive miniaturization of the hair follicle, usually with characteristic pattern distribution that occurs in genetically predisposed females and rarely with endocrine disturbances [Figure - 5]a.
 |
| Figure 5: |
Epidemiology
The actual prevalence of female patterned hair loss ranges from 6 to 64.4% and appears to be somewhat lower in Asians.[32] There are two peaks of occurrence in female patterned hair loss, 3rd and 5th decades. There is an age associated increase in the prevalence of female patterned hair loss, with 1.3% in the age group of 18-29 years, increasing to 10.3% in the seventh decade and 11.8% thereafter.[33]
Etio-pathogenesis and role of endocrine disturbances
Female patterned hair loss like male patterned hair loss is a multifactorial entity. It tends to occur in genetically pre-disposed patients with altered hair follicle cycling leading to the transformation of terminal to shorter and finer vellus hair follicles. Under the influence of sex hormones (explained above), there is progressive reduction in the size of the dermal papilla, with reduction in the anagen duration and prolongation of telogen.[32]
Evaluation
A complete gynecological history including menarche, menstrual cycle (regular / irregular), menopause, amenorrhoea, the use of oral or systemic hormonal pills, should be done to exclude influencing hormonal dysregulationsor associated underlying disorders (e.g. hormone-sensitivetumor). Examination should focus on features of hyperandrogenism (hirsutism, acne or seborrhea). Trichoscopic features including hair shaft thickness heterogeneity, thin hairs, yellow dots, perifollicular discoloration (the peripilar sign), an increased proportion of vellus hairs, and a large number of follicular units with only one emerging hair shaft are suggestive of patterned hair loss [Figure - 5]b and 5c].[34] If the history and clinical examination are indicative of androgenexcess, free androgen index has to be performed. Free testosteroneand free androgen index seem to be sensitive for the detection of hyperandrogenaemia. Free androgen index levels of 5and above are indicative for polycystic ovarian syndrome. In such a scenario, otherdisorders presenting with clinical and / or biochemical signs ofhyperandrogenism such as congenital adrenal hyperplasia, androgen-secreting tumors or Cushing syndrome need exclusion.
Clinical features and classifications
Female patterned hair loss has a chronic and a progressive course. Patients usually complain of increased thinning of hair over the vertex, butcharacteristically the frontal hairline is preserved [Figure - 5]. Early in the disease phase patients may also complain of increased shedding and hair pull test may be positive. This is because of the increasedconversion of anagen hairs to telogen. At times, an episode of telogen effluvium can unmask sub-clinical female patterned hair loss. The most widely used in classification system for female patterned hair lossis Ludwig's classification. In stage 1, there is thinning of hair from the anterior part of the crown with rarefaction of the part width (SAHA syndrome).[35] Stage II is seen with advancing age where rarefaction is more pronounced. Camouflage of the denuded areas is no longer possible.[35] In stage III is there is complete rarefaction of scalp but a fringe of frontal hair usually persists (adrenal diseases and androgen secreting tumors). Other staging systems used are Olsen and Hamilton-Norwood classification.
Treatment
Various drugs have been tried in the treatment of female patterned hair loss, but many of these treatment modalities lack sufficient evidence. There is a need for high quality studies comparing different treatment modalities for more robust data.[36]
Medical treatment
- Minoxidil: Topical minoxidil (2% or 5%) has been widely used to treat female patterned hair loss. A recent Cochrane systemic review for female patterned hair loss concluded that minoxidil treatment results in moderate increase in hair re-growth compared with placebo.[36] Minoxidil 5% twice daily was marginally better than 2% twice daily in patient assessment, but was also associated with increase adverse effects like pruritus, local irritation, and hypertrichosis.[37] In another study 5% minoxidil foam once daily was as effective and with lower rates of local intolerance than 2% minoxidil solution twice daily[38]
- Antiandrogens: Cyproterone acetate (50-100mg/day), spironolactone (100-200mg/day), flutamide (100-200mg/day) and finasteride (1 or 5mg/day) are all used in the treatment of female patterned hair loss. Vexiauet al.[39] compared cyproterone acetate (50mg) with topical 2% minoxidil and found better outcome with minoxidil. However, cyproterone acetate was more effective when clinical or biochemical evidence of hyperandrogenemia was present. In a randomized control trial comparing cyproterone acetate (50mg), finasteride (5mg) and flutamide (250mg) in hyperadrogenic alopecia, Carminaet al.[40] found improvement in Ludwig's score only in flutamide treated group.
Cosmetic concealment[32]
Camouflaging products and other concealment techniques can effectively mask areas of visible hair loss. Commonly used techniques for mild to moderate hair loss include hair fibers (keratin-made), masking lotions, topical shading, and scalp spray thickeners. For moderate–severe loss, integration hairpiece or wig may be used. Hair loss can also be covered using hats, scarves, bandanas, and turbans.
Telogen effluvium
Telogen effluvium, a common cause of hair loss is an abnormality of hair cycling that results in excessive loss of telogen hairs [Figure - 6]. The important endocrine causes of telogen effluvium are hyper and hypothyroidism, Addison's disease and hypopituitarism. In addition, thyroid disorders are associated with madarosis and multiple endocrine neoplasia type 2B is associated with disorganized eyelashes.[41]
 |
| Figure 6: A case of chronic telogen effluvium due to hypothyroidism |
Approximately 50% and 33% of patients of hyper and hypothyroidism respectively have diffuse telogen hair loss.[42] The severity of hair loss is not directly related to the severity of the endocrine abnormality and telogen effluvium can be the only presentation of sub-clinical hypothyroidism.
Telogen effluvium can be clinically suspect in patients with acute/insidious onset diffuse hair loss with positive hair pull test. Trichogram or skin biopsy, if done, shows increased in telogen hairs. Trichoscopy has limited value in diagnosis of telogen effluvium. Frequent, but not specific, findings include thepresence of empty hair follicles, a predominance of follicular units with only one hair, perifollicular discoloration (the peripilar sign), and upright re-growing hairs.[34] Early female patterned hair loss can mimic telogen effluvium, but can be differentiated on histopathology by miniaturization of hair follicles and decrease terminal to vellus hair ratio. At times, acute telogen effluvium can unmask latent female patterned hair loss.
Telogen effluvium is usually reversible, except in long-standing cases where the hair follicles are said to have atrophied. Treatment of underlying endocrine disorder is of utmost importance. Topical minoxidil 2-5% and iron supplementation in patients with low serum ferritin can be used to stimulate hair growth.[43]
Alopecia Areata
Alopecia areata is an immunologically mediated disease characterized by sudden-onset, non-scarring, patchy hair loss (involving any hair bearing area) with significant cosmetic impact [Figure - 7]. It's a fairly common disease with a lifetime risk of around 2%. Trichoscopic evaluation of early and active alopecia areata shows black dots [Figure - 8]a, exclamation hair and broken hair [Figure - 8]b, whereas longstanding inactive alopecia areata shows yellow dots and vellus hairs [Figure - 8]c.[34]
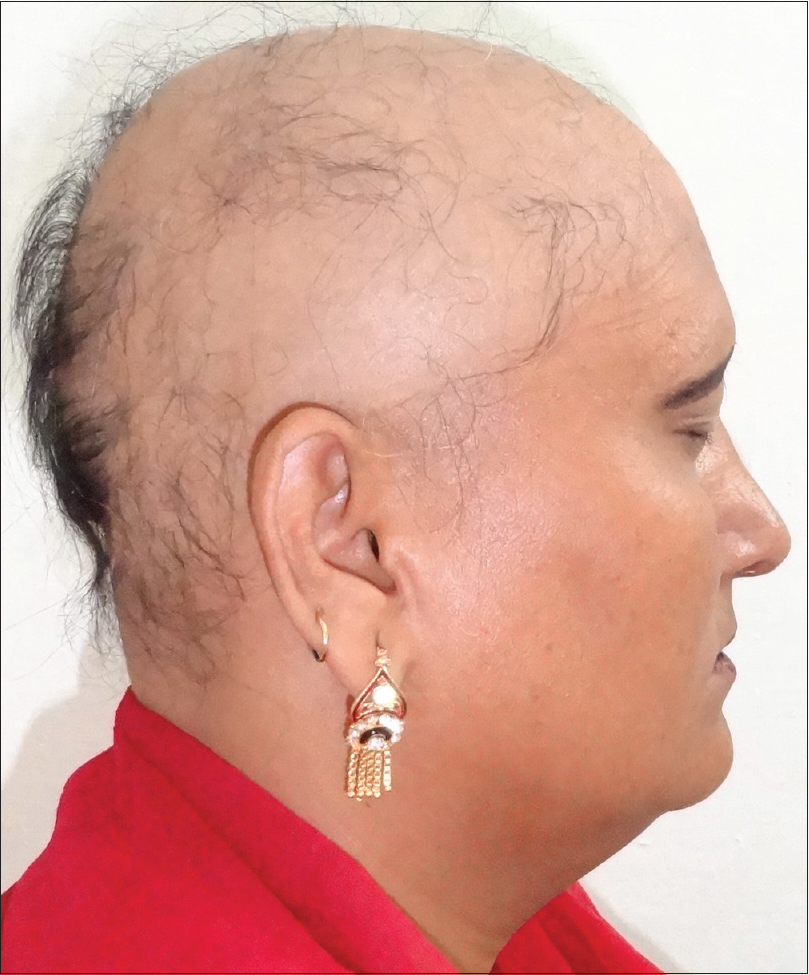 |
| Figure 7: A female patient of alopecia sub-totalis, who also suffered from hypothyroidism |
 |
| Figure 8: |
The disease has long been known to occur with various autoimmune disorders like rheumatoid arthritis, Type 1 diabetes mellitus, vitiligo, systemic lupus erythematosus, thyroiditis, pemphigus vulgaris, pernicious anemia and coeliac disease. Milgraumet al.[44] reported thyroid function test abnormalities or thyroid auto-antibodies in 24% of 45 evaluated children with alopecia areata. In adult alopecia areata patients, thyroid peroxidase antibody was found in 17.7% of cases.[45] Children with personal medical history of Down syndrome, atopy or a family medical history of thyroid diseases benefit from screening for thyroid dysfunction.[46] Screening is also recommended in patients with concerning features on physical examination such as goiter, constipation, cold intolerance, or in the patient's medical history, such as deviation on the growth chart.[46]
Treatment of alopecia areata depends on the disease extent. Limited patchy hair loss can be managed by potent topical corticosteroids or intralesional corticosteroids.[47] Extensive patchy hair loss/alopecia sub-totalis/alopecia totalis may require systemic corticosteroids and contact immunotherapy.[47]
Congenital Papular Atrichia and Vitamin-D Dependent Rickets Type II
Congenital papular atrichiais a rare autosomal recessive disorder caused due to mutation in the hairless gene, in which the scalp and body hairs are irreversibly lost soon after birth and are replaced by papular or milia like lesions over the scalp. Vitamin-D dependent rickets type II is an autosomal recessive inheritable disorder caused due to mutation in the vitamin D receptor in which target organs fail to respond to hormonal form of vitamin D. Interestingly, in addition to rickets, patients with vitamin-D receptor also manifest alopecia with few patients showing papular lesions. The alopecias associated with Vitamin-D dependent rickets type II and congenital papular atrichia show striking clinical and microscopic similarities and cannot be differentiated on the basis of clinical and histological studies.[48] Further studies suggested that this common phenotypic expression is due to their impact on common signaling pathway in normal hair growth, the product of hairless gene acting as a co-repressor of expression of vitamin D receptor.[49]
Conclusion
Hair changes are common in various endocrine diseases and can be the first clinical sign of underlying occult endocrinopathy. Dermatologist, endocrinologist and allied specialties should have knowledge of these changes for effective diagnosis and treatment.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Rosenfield RL. Hirsutism and the Variable Response of the Pilosebaceous Unit to Androgen. J Investig Dermatology Symp Proc 2005;10:205–8.
[Google Scholar]
|
| 2. |
Hamilton JB. Male hormone stimulation is prerequisite and an incitement in common baldness. Am J Anat 1942: 451.
[Google Scholar]
|
| 3. |
Bahta AW, Farjo N, Farjo B, Philpott MP. Premature Senescence of Balding Dermal Papilla Cells In Vitro Is Associated with p16INK4a Expression. J Invest Dermatol 2008; 128:1088–94.
[Google Scholar]
|
| 4. |
Futterweit W, Dunaif A, Yeh HC, Kingsley P. The prevalence of hyperandrogenism in 109 consecutive female patients with diffuse alopecia. J Am Acad Dermatol 1988; 19:831–6.
[Google Scholar]
|
| 5. |
Vexiau P, Chaspoux C, Boudou P, Fiet J, Abramovici Y, Rueda MJ. et al. Role of androgens in female-pattern androgenetic alopecia, either alone or associated with other symptoms of hyperandrogenism. Arch Dermatol Res 2000; 292:598–604.
[Google Scholar]
|
| 6. |
Orfanos CE, Adler YD, Zouboulis CC. The SAHA Syndrome. Horm Res Paediatr 2000; 54:251–8.
[Google Scholar]
|
| 7. |
Simpson D, Curran MP, Perry CM. Letrozole: a review of its use in postmenopausal women with breast cancer. Drugs 2004; 64:1213–30.
[Google Scholar]
|
| 8. |
Ohnemus U, Uenalan M, Inzunza J, Gustafsson J-Å, Paus R. The Hair Follicle as an Estrogen Target and Source. Endocr Rev 2006;27:677-706.
[Google Scholar]
|
| 9. |
Foitzik K, Krause K, Conrad F, Nakamura M, Funk W, Paus R. Human Scalp Hair Follicles Are Both a Target and a Source of Prolactin, which Serves as an Autocrine and/or Paracrine Promoter of Apoptosis-Driven Hair Follicle Regression. Am J Pathol 2006;168:748-56.
[Google Scholar]
|
| 10. |
Langan EA, Ramot Y, Goffin V, Griffiths CEM, Foitzik K, Paus R. Mind the (Gender) Gap: Does Prolactin Exert Gender and/or Site-Specific Effects on the Human Hair Follicle? J Invest Dermatol. 2010;130:886-91.
[Google Scholar]
|
| 11. |
Azziz R, Carmina E, Sawaya ME. Idiopathic Hirsutism 1. Endocr Rev 2000; 21:347–62.
[Google Scholar]
|
| 12. |
Amor KT, Rashid RM, Mirmirani P. Does D matter? The role of vitamin D in hair disorders and hair follicle cycling. Dermatol Online J 2010; 16:3.
[Google Scholar]
|
| 13. |
Madnani N, Khan K, Chauhan P, Parmar G. Polycystic ovarian syndrome. Indian J Dermatology, Venereol Leprol 2013; 79:310.
[Google Scholar]
|
| 14. |
Sharma N, Mahajan V, Jindal R, Gupta M, Lath A. Hirsutism: Clinico-investigative profile of 50 Indian patients. Indian J Dermatol 2008;53:111.
[Google Scholar]
|
| 15. |
Escobar-Morreale HF, Carmina E, Dewailly D, Gambineri A, Kelestimur F, Moghetti P, et al. Epidemiology, diagnosis and management of hirsutism: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update 2012; 18:146–70.
[Google Scholar]
|
| 16. |
Li R, Qiao J, Yang D, Li S, Lu S, Wu X, et al. Epidemiology of hirsutism among women of reproductive age in the community: a simplified scoring system. Eur J Obstet Gynecol Reprod Biol 2012; 163:165–9.
[Google Scholar]
|
| 17. |
Kopera D, Wehr E, Obermayer-Pietsch B. Endocrinology of hirsutism. Int J Trichology 2010; 2:30.
[Google Scholar]
|
| 18. |
Unluhizarci K, Kaltsas G, Kelestimur F. Non polycystic ovary syndrome-related endocrine disorders associated with hirsutism. Eur J Clin Invest 2012; 42:86–94.
[Google Scholar]
|
| 19. |
Schmidt TH, Khanijow K, Cedars MI, Huddleston H, Pasch L, Wang ET, et al. Cutaneous Findings and Systemic Associations in Women With Polycystic Ovary Syndrome. JAMA Dermatology 2016; 152:391.
[Google Scholar]
|
| 20. |
Yildiz BO, Bolour S, Woods K, Moore A, Azziz R. Visually scoring hirsutism. Hum Reprod Update 2010; 16:51–64.
[Google Scholar]
|
| 21. |
Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E. American association of clinical endocrinologists, american college of endocrinology, and androgen excess and polycystic ovarian syndrome society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome-part 1.Endocr Pract 2015; 21:1291–300.
[Google Scholar]
|
| 22. |
Hahn S, Kuehnel W, Tan S, Kramer K, Schmidt M, Roesler S, et al. Diagnostic value of calculated testosterone indices in the assessment of polycystic ovary syndrome. Clin Chem Lab Med 2007; 45:202–7.
[Google Scholar]
|
| 23. |
Iliodromiti S, Kelsey TW, Anderson RA, Nelson SM. Can Anti-Müllerian Hormone Predict the Diagnosis of Polycystic Ovary Syndrome? A Systematic Review and Meta-Analysis of Extracted Data. J Clin Endocrinol Metab 2013; 98:3332–40.
[Google Scholar]
|
| 24. |
Mofid A, Seyyed Alinaghi SA, Zandieh S, Yazdani T. Hirsutism. Int J Clin Pract 2007; 62:433–43.
[Google Scholar]
|
| 25. |
Somani N, Turvy D. Hirsutism: An Evidence-Based Treatment Update. Am J Clin Dermatol 2014; 15:247–66.
[Google Scholar]
|
| 26. |
Swiglo BA, Cosma M, Flynn DN, Kurtz DM, Labella ML, Mullan RJ, et al. Clinical review: Antiandrogens for the treatment of hirsutism: a systematic review and metaanalyses of randomized controlled trials. J Clin Endocrinol Metab 2008; 93:1153–60.
[Google Scholar]
|
| 27. |
Martin KA, Chang RJ, Ehrmann DA, Ibanez L, Lobo RA, Rosenfield RL, et al. Evaluation and Treatment of Hirsutism in Premenopausal Women: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2008; 93:1105–20.
[Google Scholar]
|
| 28. |
Ibáñez L, Oberfield SE, Witchel S, Auchus RJ, Chang RJ, Codner E, et al. An International Consortium Update: Pathophysiology, Diagnosis, and Treatment of Polycystic Ovarian Syndrome in Adolescence. Horm Res Paediatr 2017; 88:371–95.
[Google Scholar]
|
| 29. |
Shibli-Rahhal A, Van Beek M, Schlechte JA. Cushing's syndrome. Clin Dermatol 2006; 24:260–5.
[Google Scholar]
|
| 30. |
Wendelin DS, Pope DN, Mallory SB. Hypertrichosis. J Am Acad Dermatol 2003; 48:161–82.
[Google Scholar]
|
| 31. |
Sanke S, Chander R, Jain A, Garg T, Yadav P.AComparison of the Hormonal Profile of Early Androgenetic Alopecia in Men With the Phenotypic Equivalent of Polycystic Ovarian Syndrome in Women. JAMA Dermatology 2016; 152:986.
[Google Scholar]
|
| 32. |
Singal A, Sonthalia S, Verma P. Female pattern hair loss. Indian J Dermatology, Venereol Leprol 2013; 79:626.
[Google Scholar]
|
| 33. |
Wang TL, Zhou C, Shen YW, Wang XY, Ding XL, Tian S, et al. Prevalence of androgenetic alopecia in China: a community-based study in six cities. Br J Dermatol 2010; 162:843–7.
[Google Scholar]
|
| 34. |
Mubki T, Rudnicka L, Olszewska M, Shapiro J. Evaluation and diagnosis of the hair loss patient: part II. Trichoscopic and laboratory evaluations. J Am Acad Dermatol 2014; 71:431.e1-431.e11.
[Google Scholar]
|
| 35. |
Camacho-Martínez FM. Hair Loss in Women. Semin Cutan Med Surg 2009; 28:19–32.
[Google Scholar]
|
| 36. |
van Zuuren EJ, Fedorowicz Z, Carter B. Evidence-based treatments for female pattern hair loss: a summary of a Cochrane systematic review. Br J Dermatol 2012; 167:995–1010.
[Google Scholar]
|
| 37. |
Lucky AW, Piacquadio DJ, Ditre CM, Dunlap F, Kantor I, Pandya AG, et al. Arandomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol 2004; 50:541–53.
[Google Scholar]
|
| 38. |
Blume-Peytavi U, Hillmann K, Dietz E, Canfield D, Garcia Bartels N. Arandomized, single-blind trial of 5% minoxidil foam once daily versus 2% minoxidil solution twice daily in the treatment of androgenetic alopecia in women. J Am Acad Dermatol 2011; 65:1126–1134.e2.
[Google Scholar]
|
| 39. |
Vexiau P, Chaspoux C, Boudou P, Fiet J, Jouanique C, Hardy N, et al. Effects of minoxidil 2% vs. cyproterone acetate treatment on female androgenetic alopecia: a controlled, 12-month randomized trial. Br J Dermatol 2002; 146:992–9.
[Google Scholar]
|
| 40. |
Carmina E, Lobo RA. Treatment of hyperandrogenic alopecia in women. Fertil Steril 2003; 79:91–5.
[Google Scholar]
|
| 41. |
Lause M, Kamboj A, Fernandez Faith E. Dermatologic manifestations of endocrine disorders. Transl Pediatr 2017; 6:300–12.
[Google Scholar]
|
| 42. |
Harrison S, Sinclair R. Telogen effluvium. Clin Exp Dermatol 2002; 27:389–95.
[Google Scholar]
|
| 43. |
Malkud S. Telogen Effluvium: A Review. J Clin Diagnostic Res 2015. doi: 10.7860/JCDR/2015/15219.6492.
[Google Scholar]
|
| 44. |
Milgraum SS, Mitchell AJ, Bacon GE, Rasmussen JE. Alopecia areata, endocrine function, and autoantibodies in patients 16 years of age or younger. J Am Acad Dermatol 1987; 17:57–61.
[Google Scholar]
|
| 45. |
Baars MP, Greebe RJ, Pop VJM. High prevalence of thyroid peroxidase antibodies in patients with alopecia areata. J Eur Acad Dermatol Venereol 2013; 27:e137-9.
[Google Scholar]
|
| 46. |
Patel D, Li P, Bauer AJ, Castelo-Soccio L. Screening Guidelines for Thyroid Function in Children With Alopecia Areata. JAMA Dermatology 2017; 153:1307.
[Google Scholar]
|
| 47. |
Messenger AG, McKillop J, Farrant P, McDonagh AJ, Sladden M. British Association of Dermatologists' guidelines for the management of alopecia areata 2012. Br J Dermatol 2012; 166:916–26.
[Google Scholar]
|
| 48. |
Bergman R, Schein-Goldshmid R, Hochberg Z, Ben-Izhak O, Sprecher E. The alopecias associated with vitamin D-dependent rickets type IIA and with hairless gene mutations: a comparative clinical, histologic, and immunohistochemical study. Arch Dermatol 2005; 141:343–51.
[Google Scholar]
|
| 49. |
Wang J, Malloy PJ, Feldman D. Interactions of the Vitamin D Receptor with the Corepressor Hairless. J Biol Chem 2007; 282:25231–9.
[Google Scholar]
|
Fulltext Views
11,062
PDF downloads
2,687





