Translate this page into:
Nicotinamide: Mechanism of action and indications in dermatology
Correspondence Address:
Pooja Bains
Department of Skin and V.D, Sri Guru Ram Das Institute of Medical Sciences and Research, Sri Amritsar, Amritsar, Punjab
India
| How to cite this article: Bains P, Kaur M, Kaur J, Sharma S. Nicotinamide: Mechanism of action and indications in dermatology. Indian J Dermatol Venereol Leprol 2018;84:234-237 |
Introduction
Nicotinamide aka Vitamin B3 (niacinamide, nicotinic acid amide) is the pyridine 3 carboxylic acid amide form of niacin [Figure - 1]. It is a water-soluble vitamin that is not stored in the body. The main source of vitamin in diet is in the form of nicotinamide, nicotinic acid, and tryptophan.[1] The main source of niacin include meat, liver, green leafy vegetables, wheat, oat, palm kernel oil, legumes, yeast, mushrooms, nuts, milk, fish, tea, and coffee.[2],[3] The recommended daily dose of vitamin B3 in niacin equivalent is given in [Table - 1].[4]
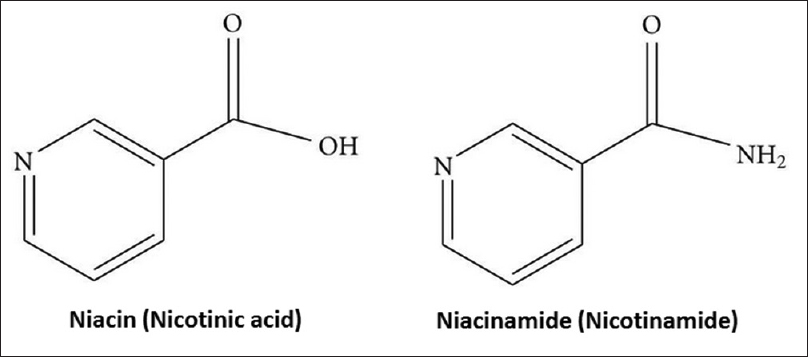 |
| Figure 1: Chemical structure of Nicotinic acid and Nicotinamide |

Metabolism of Nicotinamide
Nicotinamide is ingested in food as part of pyridine nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP) in plant and animal tissues. After the co-enzymes have separated, nicotinamide is absorbed almost completely in the small intestine. After absorption, nicotinamide is stored as NAD in the liver and excretion occurs via kidneys.[2] Tryptophan is converted to nicotinamide through kynurenine-anthranilate pathway in the liver. Tryptophan can thus satisfy the requirement for dietary nicotinic acid.[1],[5]
Biological Effects of Nicotinamide
Nicotinamide is mainly involved in the cellular energy metabolism, DNA repair, and in regulation of transcription process. The various biological effects along with the underlying mechanism of action are tabulated [Table - 2].[2],[3],[6],[7],[8],[9],[10]

Uses of Nicotinamide in Dermatological Disorders
Antiacne effect of nicotinamide
The anti-inflammatory and sebostatic role of nicotinamide plays a role in its use as an antiacne topical formulation.[11] Propionobacterium acnes colonization induces IL-8 production in acne lesions. IL-8 is a chemokine that has mitogenic activity in keratinocytes and is also involved in neutrophil chemotaxis.[3] In acne lesions, there is also activation of transcription factors NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) and activator protein 1 (AP-1), which is inhibited by nicotinamide through enzyme poly ADP ribose polymerase-1 (PARP-1) inhibition.[3] In a randomized double-blinded, controlled trial, 160 patients with moderate and predominantly inflammatory acne were given 4% nicotinamide gel or 4% erythromycin gel twice daily for 8 weeks. Both the groups reported similar regression of inflammatory lesions but the group treated with 4% nicotinamide gel had significantly greater improvement in seborrhoea scores.[12] Another double-blinded, placebo-controlled, randomized trial with 130 patients found that a 2% nicotinamide moisturiser significantly reduced sebum excretion rates when compared to a placebo moisturiser.[3] Shalita et al.[13] found reduction of inflammatory papules in 82% of those treated with 4% nicotinamide after 8 weeks of treatment. Dos et al.[14] reported that clindamycin phosphate 1% and nicotinamide gel 4% are equally and highly effective in the treatment of moderate acne either alone or in combination.
Role of nicotinamide in atopic dermatitis
Nicotinamide containing moisturisers have been shown to be effective in treatment of atopic dermatitis. In atopic dermatitis, there is a decrease in ceramides, increase in transepidermal water loss (TEWL) and impaired skin barrier. An in vitro study showed that nicotinamide caused 2–3 fold increase in free fatty acids and 1.5-fold increase in cholesterol.[9] In atopic dermatitis, there is an upregulation of aquaporin 3 that encodes water permeable channels resulting in increased water loss, which is prevented by nicotinamide.[3] In a study on 28 patients of atopic dermatitis, nicotinamide fared better than white petrolatum in decreasing TEWL.[3] In a right left comparative study in 12 male patients with dry skin, nicotinamide 2% application twice weekly for four weeks reduced TEWL by 27% in comparison to vehicle and increased stratum corneum free fatty acids and ceramides by 67% and 34%, respectively.[9] Another randomized controlled trial was done on 292 participants who received nicotinamide 500 mg twice daily or placebo for 12 months. The results showed consistent reduction in TEWL by 6–7% compared to placebo on the face and limbs.[15]
Antiageing effect of nicotinamide
Nicotinamide increases collagen production in fibroblast cultures and reduces the increased dermal glycoaminoglycosides in photodamaged skin.[16]
Nicotinamide also increases the production of the epidermal proteins keratin, filaggrin, and involucrin.[17] The glycation between protein and sugar resulting in formation of cross linked products gives a yellow colour to the skin. As nicotinamide is a precursor of antioxidant NADPH, it has antiglycation effects, thus preventing sallowing of skin.[16] In a double-blinded, split face, randomized controlled trial, 5% nicotinamide cream was compared to “vehicle only cosmetic” in 30 Japanese women on face for 8 weeks. There was a significant decrease in wrinkles and skin roughness with nicotinamide.[16]
Nicotinamide for skin lightening
As nicotinamide inhibits melanosome transfer from melanocytes to keratinocytes, it is used as a lightening agent. A double-blinded, randomized controlled trial with 202 patients found that a topical formulation containing 2% N-acetyl glucosamine and 4% nicotinamide significantly reduced the detectable area of facial spots and the appearance of pigmentation compared to vehicle formulation.[18]
Role of nicotinamide in autoimmune skin disorders
The proposed hypothesis behind use of nicotinamide in auto-immune vesiculobullous diseases is the anti-inflammatory effect of nicotinamide. It inhibits cytokines such as IL-1β, IL-6, IL-8, TNF.[3] The most studied use of nicotinamide is in bullous pemphigoid. One case report suggests that nicotinamide monotherapy in dose of 1.5 g/day is an effective treatment in localized bullous pemphigoid.[3]
A randomized, open-label clinical trial compared nicotinamide 500 mg thrice daily plus tetracycline 500 mg four times a day to prednisone 40 to 80 mg a day in 18 patients with bullous pemphigoid. In the nicotinamide and tetracycline group, 5 out of 12 patients had complete responses, compared with one of the 6 patients in the prednisone group. The results point towards a significant effect of nicotinamide and tetracycline therapy in comparison to prednisone in selected patients. There were decreased adverse effects in the nicotinamide and tetracycline group.[19] In another double-blinded, controlled trial in eight pemphigus vulgaris patients, 60 lesions were randomized to either nicotinamide 4% gel or placebo gel for 30 days. It was seen that the mean epithelialisation index for the skin lesions that received nicotinamide was significantly higher.[20] There are various case reports of successful use of nicotinamide and tetracyclines in other autoimmune blistering disorders including cicatricial pemphigoid, lichen planus pemphigoides, dermatitis herpetiformis, and immunoglobulin A bullous dermatosis. Nicotinamide can thus be used as a steroid sparing agent.
Anticarcinogenic and photoprotective effect of nicotinamide
The enzyme PARP-1, which is inhibited by nicotinamide, is involved in cell senescence, ageing, and cancer. The excessive activation of PARP-1 by UV rays result in depletion of cellular NAD, which further cause glycolytic failure leading to cell necrosis.[21] Nicotinamide replenishes cellular energy as it is a precursor of NAD and NADP. Secondly, it prevents overactivation of PARP-1 by negative feedback, thus preventing cell senescence. Besides, nicotinamide protects against UVA and UVB induced immunosuppression. In a phase 3 clinical trial, the administration of nicotinamide 500 mg twice daily led to a statistically significant reduction in the number of cases of non melanoma skin cancers and actinic keratosis though the effect disappeared 6 months after stopping nicotinamide.[22] This is an area of further research but nicotinamide might prove beneficial in prevention of skin cancers.
Treatment of pellagra
Nicotinamide has been named PP factor or Vitamin PP due to its role in treating pellagra and was first used clinically in 1937.[8] Pellagra is characterized by triad of diarrhoea, dermatitis, and dementia. Pellagra is caused because of cellular deficiency of niacin, resulting from an inadequate dietary supply of niacin and tryptophan mostly seen in chronic alcoholics, and in patients with gastrointestinal diseases or severe psychiatric disturbances.[23] Rare causes are functioning carcinoid tumors and Hartnup disease, therapy with isoniazid, 6-mercaptopurine, or 5-fluorouracil.[24] The cutaneous changes include erythema and scaling on sun exposed areas with a “butterfly” eruption on face and a well-marginated eruption on the front of the neck (“Casal's necklace”).[24] Oral nicotinamide is given initially as 100–300 mg daily in 3–4 doses until improvement in acute symptoms, then slowly tapered to 50 mg BD or TDS.[23] Oral nicotinamide is preferred over niacin/nicotinic acid as it does not precipitate flushing, itching and burning commonly seen with ingestion of niacin in large doses.[24]
Nicotinamide as anti-psoriatic agent
Nicotinamide has a potential role in the treatment of psoriasis because of its anti-inflammatory effect, inhibition of the expression of ICAM-1 and MHC-II, and production of IL-12, TNF- α, and IL-1. The nicotinamide-methotrexate combination may prove to be superior to methotrexate alone in the treatment of psoriasis.[25]
In a randomized controlled trial, the combination of topical calcipotriene and nicotinamide was more effective than either of the two as monotherapy as a steroid sparing topical treatment. The addition of nicotinamide as an adjuvant to calcipotriene can enhance the efficacy of calcipotriene when used for topical psoriasis treatment.[26]
Adverse Effects and Safety Profile
Nicotinamide is a safe and inexpensive compound with negligible side effects. It is well tolerated even in doses of 1 g/day to 3 g/day.[3] There are no reports of teratogenicity with nicotinamide. Minor side effects include nausea, vomiting, headache, fatigue. It does not cause vasodilatory side effects like flushing, alteration in blood pressure, body temperature or pulse as seen with niacin.[3] In topical formulation, it does not cause skin irritation, photosensitization in concentrations of 0.0001% to 4%.[3]
Conclusion
The existing clinical data and literature on nicotinamide suggests that it is an inexpensive, safe drug with beneficial effects as an adjunct in many dermatological diseases because of its anti-inflammatory, anti-oxidant, barrier repair and protective effects. It can be used both as a topical and oral drug without any major adverse effects.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Aguilera-Méndez A, Fernández-Lainez C, Ibarra-González I, Fernandez-Mejia C. The chemistry and biochemistry of niacin (B3). In: Preedy VR, editor. B Vitamins and Folate: Chemistry, Analysis, Function and Effects. Cambridge: RSC Publishing; 2012. p. 108-26.
[Google Scholar]
|
| 2. |
Wohlrab J, Kreft D. Niacinamide-mechanisms of action and its topical use in dermatology. Skin Pharmacol Physiol 2014;27:311-5.
[Google Scholar]
|
| 3. |
Surjana D, Damian DL. Nicotinamide in dermatology and photoprotection. Skinmed 2011;9:360-5.
[Google Scholar]
|
| 4. |
ICMR. Nutrition Requirement and Recommended Dietary Allowances for Indians, A Report of the Expert Group of the ICMR; 2010.
[Google Scholar]
|
| 5. |
DiPalma JR, Thayer WS. Use of niacin as a drug. Annu Rev Nutr 1991;11:169-87.
[Google Scholar]
|
| 6. |
Ungerstedt JS, Blömback M, Söderström T. Nicotinamide is a potent inhibitor of proinflammatory cytokines. Clin Exp Immunol 2003;131:48-52.
[Google Scholar]
|
| 7. |
Fivenson DP. The mechanisms of action of nicotinamide and zinc in inflammatory skin disease. Cutis 2006;77:5-10.
[Google Scholar]
|
| 8. |
Murray MF. Nicotinamide: An oral antimicrobial agent with activity against both Mycobacterium tuberculosis and human immunodeficiency virus. Clin Infect Dis 2003;36:453-60.
[Google Scholar]
|
| 9. |
Tanno O, Ota Y, Kitamura N, Katsube T, Inoue S. Nicotinamide increases biosynthesis of ceramides as well as other stratum corneum lipids to improve the epidermal permeability barrier. Br J Dermatol 2000;143:524-31.
[Google Scholar]
|
| 10. |
Namazi MR. Nicotinamide as a potential addition to the anti-atopic dermatitis armamentarium. Int Immunopharmacol 2004;4:709-12.
[Google Scholar]
|
| 11. |
Namazi MR. Nicotinamide in dermatology: A capsule summary. Int J Dermatol 2007;46:1229-31.
[Google Scholar]
|
| 12. |
Weltert Y, Chartier S, Gibaud C, Pechenart P, Girard F, Courau S, et al. Double blind clinical evaluation of the efficacy of nicotinamide gel versus 4% erythromycin gel in the treatment of moderate acne in predominantly inflammatory componenet. Les Nouv Dermatologiques 2004;23:385-94.
[Google Scholar]
|
| 13. |
Shalita AR, Smith JG, Parish LC, Sofman MS, Chalker DK. Topical nicotinamide compared with clindamycin gel in the treatment of inflammatory acne vulgaris. Int J Dermatol 1995;34:434-7.
[Google Scholar]
|
| 14. |
Dos SK, Barbhuiya JN, Jana S, Dey SK. Comparative evaluation of clindamycin phosphate 1% and clindamycin phosphate 1% with nicotinamide gel 4% in the treatment of acne vulgaris. Indian J Dermatol Venereol Leprol 2003;69:8-9.
[Google Scholar]
|
| 15. |
Chen AC, Martin AJ, Dalziell RA, Halliday GM, Damian DL. Oral nicotinamide reduces transepidermal water loss: A randomized controlled trial. Br J Dermatol 2016;175:1363-5.
[Google Scholar]
|
| 16. |
Kawada A, Konishi N, Momma T, Oiso N, Kawara S. Evaluation of anti-wrinkle effects of a novel cosmetic containing retinol using the guideline of the Japan cosmetic industry association. J Dermatol 2009;36:583-6.
[Google Scholar]
|
| 17. |
Oblong JE, Bissett DL, Ritter JL, Kurtz KK, Schnicker MS. Effect of Niacinamide on Collagen Synthesis and Markers of Keratinocyte Differentiation. 60th Annual Meeting of the American Academy of Dermatology, New Orleans; 2002.
[Google Scholar]
|
| 18. |
Kimball AB, Kaczvinsky JR, Li J, Robinson LR, Matts PJ, Berge CA, et al. Reduction in the appearance of facial hyperpigmentation after use of moisturizers with a combination of topical niacinamide and N-acetyl glucosamine: Results of a randomized, double-blind, vehicle-controlled trial. Br J Dermatol 2010;162:435-41.
[Google Scholar]
|
| 19. |
Khandpur S, Verma P. Bullous pemphigoid. Indian J Dermatol Venereol Leprol 2011;77:450-5.
[Google Scholar]
|
| 20. |
Iraji F, Banan L. The efficacy of nicotinamide gel 4% as an adjuvant therapy in the treatment of cutaneous erosions of pemphigus vulgaris. Dermatol Ther 2010;23:308-11.
[Google Scholar]
|
| 21. |
Damian DL. Photoprotective effects of nicotinamide. Photochem Photobiol Sci 2010;9:578-85.
[Google Scholar]
|
| 22. |
Chen AC, Martin AJ, Choy B, Fernández-Peñas P, Dalziell RA, McKenzie CA, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med 2015;373:1618-26.
[Google Scholar]
|
| 23. |
Srinivas CR, Sekar CS, Jayashree R. Photodermatoses in India. Indian J Dermatol Venereol Leprol 2012;78 Suppl 1:S1-8.
[Google Scholar]
|
| 24. |
Sarkany RP, Breathnach SM, Morris AA, Weismann K, Flynn PD. Metabolic and nutritional disorders. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. 8th ed. Oxford:Wiley-Blackwell; 2010. p. 59.63.
[Google Scholar]
|
| 25. |
Namazi MR. Nicotinamide: A potential addition to the anti-psoriatic weaponry. FASEB J 2003;17:1377-9.
[Google Scholar]
|
| 26. |
Forbat E, Al-Niaimi F, Ali FR. Use of nicotinamide in dermatology. Clin Exp Dermatol 2017;42:137-44.
[Google Scholar]
|
Fulltext Views
30,578
PDF downloads
9,032





