Translate this page into:
Penile Mycobacterium avium complex spindle cell pseudotumor
2 Department of Pathology, Ramon Y Cajal University Hospital, Madrid, Spain
3 Department of Internal Medicine, Ramon Y Cajal University Hospital, Madrid, Spain
Correspondence Address:
Pablo Fonda-Pascual
Department of Dermatology, Ramon Y Cajal University Hospital, Madrid
Spain
| How to cite this article: Fonda-Pascual P, Arana-Raja A, Moreno-Arrones OM, Buendia-Castaño D, Garcia Del Real CM, Diz-Fariña S. Penile Mycobacterium avium complex spindle cell pseudotumor. Indian J Dermatol Venereol Leprol 2018;84:199-202 |
Sir,
A 50-year-old man with a 3-years history of late-onset combined immunodeficiency, associated with persistent hypogammaglobulinemia, total lymphopenia with anergy of T4 cell response, and insufficient generation of interferon presented with lesions on the genital area. The patient denied local symptoms such as pain or dysuria, or relevant systemic symptoms such as fever or asthenia. He had only slight pruritus around the lesions. He was previously treated with oral fluconazole for suspected genital mucosal candidiasis without any improvement.
His past medical history also included a generalized Mycobacterium avium complex infection, transfusion-related hyperferritinemia, type-2 diabetes, and mixed polyneuropathy. His medications included azithromycin, rifabutin, trimethoprim/sulfamethoxazole, valsartan, hydrochlorothiazide, metformin, omeprazole, and vitamin-B complex. He denied taking any over-the-counter medications or any new drug. The patient was nonalcoholic and a nonsmoker.
Cutaneous examination revealed several yellowish firm papules located on the shaft of the penis and also on the free edge of the foreskin. A 1 × 1 cm-indurated ulcer with mamelonated borders and a fibrinous base was present on the uppermost foreskin [Figure - 1]. There was neither lymphadenopathy nor lymphangitis.
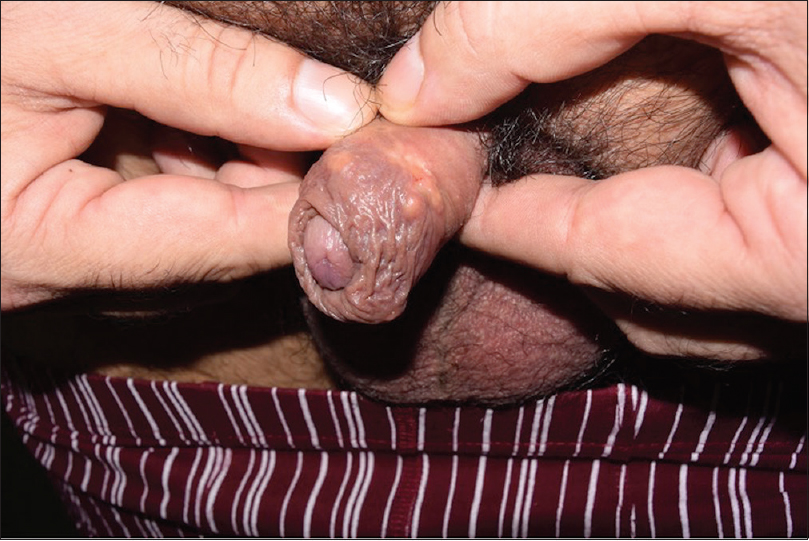 |
| Figure 1a: There are several (5–6) 0.5-cm yellowish papules on the whole surface of the foreskin, stony to touch, and detached from the deep tissues |
An incisional biopsy from the edge of the ulcer was performed for histological and microbiological study. Under microscopic examination, orthokeratotic epidermal acanthosis was observed over a diffuse spindle cell proliferation, occupying the whole dermis, without destruction of the preexisting appendages. This proliferation was composed of spindle cells with oval vesicular nuclei, while others were more hyperchromatic and wavy. They were grouped in small fascicles, haphazardly arranged, in a storiform pattern, with a diffuse extension. No mitotic figures or gross pleomorphism were observed. There were scattered inflammatory cells composed of lymphocytes and neutrophils [Figure - 2]. The main spindle cells showed CD31, lysozyme, CD68, factor XIIIa, and D2-40 positivity. Additional immunohistochemistry was performed revealing negativity to CKAE1-AE3, smooth muscle actin, desmin, and human herpes virus-8. Ziehl-Neelsen staining showed abundant acid-fast bacteria [Figure - 3]. With these findings, along with the referred clinical features, a diagnosis of Mycobacterium avium complex cutaneous involvement with fusocellular pseudotumoral pattern was established.
 |
| Figure 1b: A round 1 cm ulcer with granulomatous borders on the uppermost foreskin is observed adjacent to the aforementioned papules |
 |
| Figure 2a: There is a diffuse spindle cell infiltrate that involves the whole dermis without effacing the adnexal structures. Orthokeratotic parakeratosis with acanthosis is observed, with focal ulceration at the right side of the biopsy [hematoxylin and eosin (H and E), ×40] |
 |
| Figure 2b: There is a dense infiltrate of spindle cells in a storiform pattern with interspersed lymphocytes (H and E, ×100) |
 |
| Figure 2c: Higher magnification picture of the epidermis and papillary dermis showing the prominent hyperkeratosis and a diffuse infiltrate of mixed cells such as histiocytes, granulocytes, and lymphocytes (H and E, ×200) |
 |
| Figure 2d: Fusocellular infiltrate with interspersed collagen bundles (H and E, ×400) |
 |
| Figure 3a: Immunohistochemistry showing spindle cell infiltrate with prominently CD31-positive, CD68-positive |
 |
| Figure 3b: The spindle cell infiltrate is prominently CD31-positive |
 |
| Figure 3c: After performing Ziehl-Neelsen staining, a large number of acid-fast bacilli could be observed with some prominent foci Ziehl-Neelsen × 400, highlighted with arrows) |
 |
| Figure 3d: Many identifiable bacilli at a greater magnification (Ziehl-Neelsen, ×600) |
The first cutaneous involvement by atypical mycobacteria with fusocellullar pseudotumor pattern was first described by Dr. Wood in 1985.[1] This pattern mimicked the histoid variant of lepromatous leprosy,[2] so Wood published it as a “histoid” pseudotumoral variety of M. avium complex. In time, this pattern of mycobacterial infection has been documented more frequently in different organs,[3] with the name of mycobacterial fusocellular pseudotumour.[4] In the skin, most of the cases occur in immunosuppressed individuals, and this complex [previously Mycobacterium avium intracellulare complex] is the most common mycobacteria that can cause it, although other acid-fast bacilli and even other microorganisms have been described as causative agents for the pseudotumoral pattern.[5] It is considered that these lesions may occur due to a decrease of interferon-γ production by CD4+ T-cell lymphocytes.[4],[5] Mycobacterial spindle cell pseudotumor has been mostly described in middle-aged males as in the case we present.[5] The differential diagnosis should include other fusocellular proliferations, depending on whether the involvement is more superficial or deep, or how well defined they are, dermatofibroma, inflammatory myofibroblastic tumor, or nodular fasciitis should be considered.[4] Due to its morphology, dermatofibroma might look similar; however, this tumor shows thick collagen bundles trapped in its periphery, a lack of neutrophilic infiltrate and factor XIIIa positivity in immunohistochemistry, which is usually negative in mycobacterial pseudotumor.[3] Inflammatory myofibroblastic tumor, which has prominent blood vessels and hyalinized areas alternating with spindle cells, lacks neutrophils and shows positivity for muscle markers in the immunohistochemistry;[3] finally in immunosuppressed patients, a tumor-stage Kaposi sarcoma should also be ruled out.[5]
In summary, in immunocompromised patients, atypical mycobacterial infections must be included in the differential diagnosis of spindle cell neoplasms,[4] especially when they present as neutrophil aggregates, but it is important to consider it, even if these cells are scarce or absent, because a correct diagnosis will lead to an appropriate treatment and will decrease the risk of dissemination of the infection. With respect to late-onset combined immunodeficiency and related disorders, there is no data of associated atypical mycobacterial opportunistic infections including Mycobacterium avium complex.[4]
Regarding the case we present, cultures were positive for M. avium var. Colombiensis (demonstrated by automated Accuprobe® Mycobacterium avium identification test), displaying sensitivity to macrolides and ethambutol (objectivized through BACTEC liquid media); therefore, therapy was promptly initiated with azithromycin 500 mg/12 h and ethambutol 800 mg/24 h. After 2 months of follow-up, the patient developed an aggressive nodal anaplastic large T-cell lymphoma secondary to his underlying immunodeficiency, dying shortly after the diagnosis. An autopsy to establish the exact death cause was not performed as the relatives were unwilling. This outcome seemed to be related with the underlying condition of the patient because immunosuppression may lead to lymphoproliferative disorders. Nevertheless, we did not include this lymphoma in the differential diagnosis because there were several lesions, and the lymphoma was ALK+, which suggests a primary nodal origin.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understand that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Wood C, Nickoloff BJ, Todes-Taylor NR. Pseudotumor resulting from atypical mycobacterial infection: A “histoid” variety of Mycobacterium avium-intracellulare complex infection. Am J Clin Pathol 1985;83:524-7.
[Google Scholar]
|
| 2. |
Mathur M, Jha A, Joshi R, Wagle R. Histoid leprosy: A retrospective clinicopathological study from central Nepal. Int J Dermatol 2017;56:664-8.
[Google Scholar]
|
| 3. |
Yeh I, Evan G, Jokinen CH. Cutaneous mycobacterial spindle cell pseudotumor: A potential mimic of soft tissue neoplasms. Am J Dermatopathol 2011;33:e66-9.
[Google Scholar]
|
| 4. |
Umlas J, Federman M, Crawford C, O'Hara CJ, Fitzgibbon JS, Modeste A, et al. Spindle cell pseudotumor due to Mycobacterium avium-intracellulare in patients with acquired immunodeficiency syndrome (AIDS). Positive staining of mycobacteria for cytoskeleton filaments. Am J Surg Pathol 1991;15:1181-7.
[Google Scholar]
|
| 5. |
Shiomi T, Yamamoto T, Manabe T. Mycobacterial spindle cell pseudotumor of the skin. J Cutan Pathol 2007;34:346-51.
[Google Scholar]
|
Fulltext Views
1,641
PDF downloads
1,535





