Translate this page into:
Atorvastatin as adjunctive therapy for chronic plaque type psoriasis versus betamethasone valerate alone: A randomized, double-blind, placebo-controlled trial
Correspondence Address:
Sharlene Helene H. Chua
Unit 405, 711 Camba Street, Binondo, Manila
Philippines
| How to cite this article: Chua SH, Tioleco GM, Dayrit CA, Mojica WP, Dofitas BL, Frez LF. Atorvastatin as adjunctive therapy for chronic plaque type psoriasis versus betamethasone valerate alone: A randomized, double-blind, placebo-controlled trial. Indian J Dermatol Venereol Leprol 2017;83:441-447 |
Abstract
Background: Psoriasis is a T helper 1 cell-mediated chronic inflammation. Statins have been found to have anti-inflammatory and immunomodulatory effects targeting T helper 1 cells and thus, are being investigated as treatments for psoriasis.Aims: To investigate the efficacy and safety of atorvastatin as adjunctive treatment for mild to moderate chronic plaque psoriasis; and the impact of atorvastatin on quality of life. The study also aimed to correlate the beneficial effects of atorvastatin with its lipid-lowering effects.
Methods: Twenty-eight (19–65 year old) mild-moderate chronic plaque psoriasis patients were randomly assigned to two groups (treatment group: atorvastatin 40 mg OD; control group: placebo OD) and followed up for 6 months. All were allowed to use betamethasone valerate 0.1% ointment twice a day for a maximum of 3 weeks continuous application with 1-week rest periods in between. Primary outcome measures were the mean percentage reduction in Psoriasis Area and Severity Index (PASI) scores and percentage of patients achieving PASI-50.
Results: Fourteen patients (treatment: 6, control: 8) completed the trial. Mean reductions in PASI scores between the treatment (2.15 ± 2.17) and control (1.69 ± 2.36) groups were not statistically significant (P = 0.636). Intention-to-treat analysis of PASI-50 showed increased risk of treatment failure with atorvastatin as adjunct but estimates were not significant. Changes in Dermatology Life Quality Index (DLQI) scores (P = 0.214) and high-sensitivity C-reactive protein (P = 0.884) were likewise not statistically significant. Reductions in PASI scores were not linearly correlated with reductions in total cholesterol (P = 0.924), triglycerides (P = 0.274), low-density lipoprotein-cholesterol (P = 0.636), high-density lipoprotein-cholesterol (P = 0.584), or high-sensitivity C-reactive protein levels (P = 0.906). Adverse effects in the treatment group were transient elevated transaminases (n = 1) and mild myalgia (n = 1).
Limitations: A 50% dropout rate was experienced. This remarkably high dropout rate decreases the robustness of the study results.
Conclusions: Although atorvastatin exhibited earlier percentage reduction in PASI scores, it was not able to produce an additional benefit compared to psoriatic patients applying steroid alone.
Introduction
Psoriasis is a chronic debilitating skin disease characterized by T helper 1 and T helper 17 cell-mediated inflammation. It continues to burden about 0.1%–11.8% of the worldwide population.[1] Management must be individually tailored with consideration given to the extent of disease, the patient's quality of life, benefits and potential side effects.
Statins are 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibitors and aside from treating dyslipidemia, have been found to have pleiotropic anti-inflammatory and immunomodulatory effects.[2],[3],[4],[5] As a result of these effects, they have been recently investigated for their possible adjunctive efficacy in the treatment of psoriasis.[6],[7],[8],[9],[10],[11] They are found to essentially target inflammation by inhibiting lymphocyte function-associated antigen-1, a T-cell surface protein that interacts with antigen-presenting cells. Inhibition of lymphocyte function-associated antigen-1 leads to the inhibition of activation and migration of T-cells to the skin, thus decreasing production of pro-inflammatory cytokines such as interferon-γ, tumor necrosis factor-α and interleukin-1β. This results in the inhibition of keratinocyte activation and proliferation.[12] Various clinical trials have been conducted to investigate the efficacy of statins as treatments for psoriasis, but most of these studies had small sample sizes, were open-labelled, had different baseline Psoriasis Area and Severity Index (PASI) scores, or produced contradictory results.[6],[7],[8],[9],[10],[11] Because of this paucity of well-designed randomized controlled trials to investigate the efficacy of statins as adjuncts to anti-psoriatic therapy, this study was undertaken. Impact of atorvastatin on quality of life, as well as the correlation of its potential anti-inflammatory effects and lipid-lowering effects, was also investigated.
Methods
This was a single-center, parallel-group, randomized, double-blind, placebo-controlled clinical trial that was conducted for 9 months at a dermatology out-patient clinic in a tertiary hospital in the Philippines.
Sample size determination was done based on the data from the trial by Vasiuk et al. with the mean changes in PASI scores of 16.99 (standard deviation: 7.24) in the treatment group versus 6.27 (standard deviation: 4.57) in the control group and 95.8% of the atorvastatin group versus 13.3% in the control group achieving PASI-50 after 6 months. A desired power of 90% and alpha error probability of 5% was employed, resulting into a needed sample size of 11 patients in each arm. To account for dropouts, 14 patients were recruited into each arm. Recruitment started on February 2013 and patients were followed up for 6 months. Final data collection was completed on October 2013.
Twenty-eight patients aged 19–65 years assessed to have mild-to-moderate chronic plaque psoriasis with PASI scores <10, were enrolled into the study and randomized into two equal treatment groups. Before participating in the study, patients were required to have a washout period of psoriasis pharmacotherapy for at least 2 months for phototherapy and systemic drugs and 2 weeks for topical therapies.
Patients with uncontrolled hypertension, endocrine or other metabolic diseases; with known allergy to any of the treatments; active liver disease or liver enzymes thrice the upper limit; any myopathy or presence of elevated creatine kinase-MM levels; patients taking any drug that might interact with statins and those already taking statins or with clear indications for statin treatment; impaired renal function or creatinine >2.0 mg/dL; active infection or white blood cell >10,000/cmm and pregnant or lactating women were excluded from the study. The study was approved by the Ethics Committee and Institutional Review Board. Informed consent was obtained from all participants at study entry.
Patients were randomly assigned into two groups through a computer-generated randomization table with sequencing of assignments unknown to the primary investigator. The assigned interventions were placed in sequentially-numbered, opaque envelopes which were opened by one of the secondary investigators only after the patient had agreed to participate in the study. Patients were assigned numerical codes that were indicated in their case record forms.
Primary and secondary efficacy parameters and safety parameters
Primary efficacy parameters included mean gross and percentage reduction in PASI scores from baseline to the end of 6 months, and percentage of patients achieving PASI-50 in each arm at the end of 6 months.
The Psoriasis Area and Severity Index (PASI) is the most widely used assessment tool for psoriasis severity in clinical trials. It was developed by Fredriksson and Pettersson back in 1978 and involves grading psoriatic plaques based on erythema (E), infiltration or thickness (I), and desquamation or scaling (D). PASI-50 is defined as at least 50% reduction in PASI score from baseline.
Secondary efficacy parameters included the following: monthly mean change in PASI scores, percentage of patients achieving PASI-50 at the end of 3 months, mean change in Dermatology Life Quality Index (DLQI) scores after 6 months, mean change in lipid profile levels, mean change in high-sensitivity C-reactive protein (hsCRP) levels, and adverse events.
The Dermatology Life Quality Index (DLQI) is a 10-item self-administered questionnaire developed by Finlay and Khan in 1994 designed to evaluate the quality of life of patients with skin diseases. A study comparing the DLQI with the Psoriasis Disability Index concluded that the two questionnaires were equivalent.[13] A validated Filipino version of the DLQI questionnaire was used for this study.[14]
Fourteen patients took atorvastatin 40 mg once a day, while 14 patients took a similar-looking placebo tablet once a day. The study duration was 6 months. All patients were allowed to use betamethasone valerate 0.1% ointment twice a day, for a maximum of 3 weeks continuous application with 1-week rest periods in between, for the duration of the study. Drug dispensing was done by a secondary investigator, while clinical assessment was done by the primary investigator who was blinded to the treatment assignments.
Patients' PASI scores, lipid profiles, aspartate aminotransferase (AST), alanine aminotransferase (ALT), high-sensitivity C-reactive protein (hsCRP) levels and Dermatology Life and Quality Index (DLQI) scores were taken at baseline. The primary investigator recorded the PASI scores for every visit. Recording of the lipid profile, AST and ALT values was done by another secondary investigator so that the primary investigator would not be biased by the trend of the said parameters. Photo-documentation was done throughout the study. Patients were also asked to bring their medications at each visit to check for compliance. PASI scores, lipid profiles, AST and ALT levels were monitored monthly, while DLQI scores and hsCRP levels were re-evaluated after 6 months of therapy. Difference in the mean changes in PASI scores, lipid profile levels, DLQI scores and hsCRP levels between groups was compared. Difference in the proportion of patients reaching PASI-50 after 3 months and after 6 months of therapy was compared. Correlation between changes in PASI scores and changes in lipid profile levels, as well as correlation between changes in PASI scores and changes in hsCRP levels were computed.
Adverse event monitoring was by active query and spontaneous reporting starting from day 1 of receiving the study drug until their last follow-up.
Intention-to-treat analysis was the primary efficacy analysis. Patients included were those who had at least one assessment beyond baseline (month 1). The last measurement of each randomized patient was moved forward to represent the end-of-treatment measurement at 6 months. Per-protocol analysis was the secondary efficacy analysis. All data analyses were performed using a statistical software STATA 12.0 (StataCorp, College Station, Texas, USA).
Partial funding was given by the Philippine Dermatological Society as a research grant. The primary investigator shouldered the rest of the expenses. The authors state no conflicts of interest.
Results
Twenty-eight patients complied with the study criteria and entered the study. A total of 9 (32.1%) patients were lost to follow-up, 4 from the atorvastatin group after 1 month of treatment and 5 from the control group (one patient completed 1 month of treatment and another completed 3 months). In the atorvastatin group, three patients withdrew consent after 2 weeks and one had to be excluded after 1 month of treatment due to noncompliance to study procedures. In the placebo group, one patient was withdrawn after 4 months of treatment due to increased PASI score of >10 and increasing low-density lipoprotein levels warranting statin initiation. Therefore, 14 patients completed the trial with six patients from the atorvastatin group and eight patients from the placebo group [Figure - 1]. Baseline characteristics of both groups showed no statistically significant differences in terms of age, sex, body mass index, blood pressure, baseline PASI scores, lipid profile levels, AST, high-sensitivity C-reactive protein and DLQI scores [Table - 1].
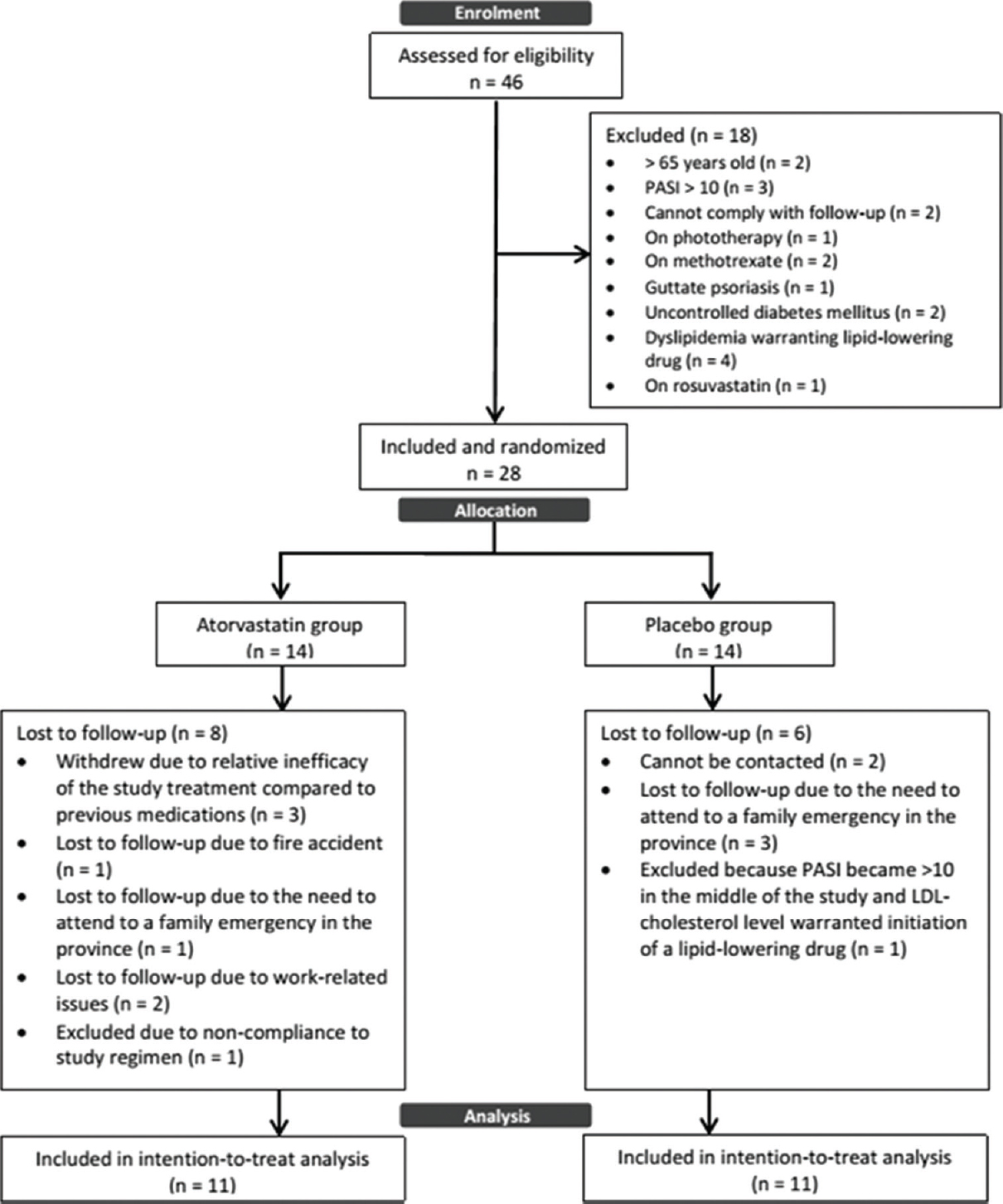 |
| Figure 1: Flowchart of study participants |

Efficacy analysis
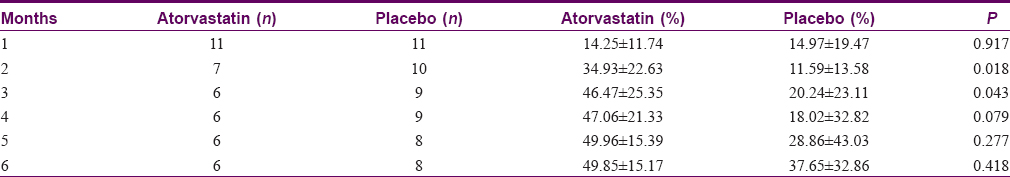
Eleven patients in each arm had at least one measurement beyond baseline and thus were included in the intention-to-treat analysis. After 6 months, mean reductions in PASI scores in the atorvastatin group were higher than those of the placebo group [Figure - 2]. However, none achieved statistical significance in both the intention-to-treat [Table - 2] and per-protocol analysis. Mean percentage reductions in PASI scores were also higher for the atorvastatin group compared to the placebo group but none achieved statistical significance in the intention-to-treat analysis. Using per-protocol analysis, statistical significance was noted between the two groups for month 2 (P = 0.018) and month 3 (P = 0.043), favoring atorvastatin [Table - 3].
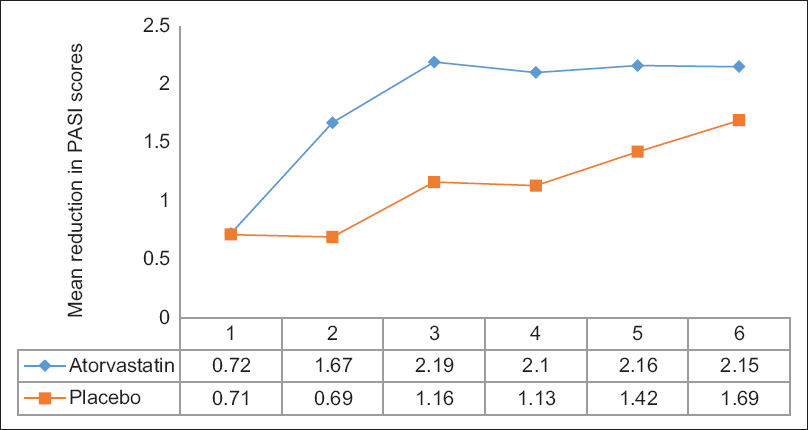 |
| Figure 2: Mean reductions in Psoriasis Area and Severity Index scores over 6 months of therapy (intention-to-treat) |

For the therapeutic effect, treatment success was defined as at least 50% reduction in PASI scores from baseline and treatment failure as < 50% reduction in PASI scores from baseline. In the atorvastatin group, 3 (27.3%) patients were able to achieve treatment success as compared to 5 (45.5%) patients in the placebo group [Table - 4]. Intention-to-treat analysis showed a trend to increased risk of treatment failure with atorvastatin as an adjunct. However, these estimates (relative risk reduction, absolute risk reduction) were not precise and not significant (null value included in the 95% confidence interval) as shown in [Table - 5]. Patients from the atorvastatin group [Figure - 3] and placebo group [Figure - 4] showed good to marked improvement at the end of treatment.


 |
| Figure 3: Lesions on the thigh of a patient from the atorvastatin group. (a) at baseline. (b) at the third month. (c) sustained improvement at six months of follow-up |
 |
| Figure 4: Lesions on the back of a patient from the placebo group. (a) at baseline. (b) at the third month. (c) sustained improvement at six months of follow-up |
Reduction in lipid profile levels and inflammatory markers
The atorvastatin group had significantly lower total cholesterol and low-density lipoprotein-cholesterol levels than the placebo group. It was also observed that hsCRP levels did not decrease with treatment and even increased with a mean of 7.58 (standard deviation: 32.92, confidence interval: −42.14–26.17) in the atorvastatin group versus 5.14 (standard deviation: 28.34, confidence interval: −28.83–18.56) in the placebo group. The difference was not statistically significant (P = 0.884).
Using Pearson's correlation coefficient, reductions in PASI scores were not linearly correlated with reductions in total cholesterol (r = −0.030, P = 0.924), triglycerides (r = 0.328, P = 0.274), low-density lipoprotein-cholesterol (r = −0.145, P = 0.636), high-density lipoprotein-cholesterol (r = 0.168, P = 0.584) or hsCRP levels (r = −0.035, P = 0.906).
Change in Dermatology Life and Quality Index scores
At the end of the 6-month treatment period, the mean reduction in DLQI scores in the atorvastatin group (mean: 6.5, standard deviation: 5.58, confidence interval: 0.65–12.35) was higher than in the placebo group (mean: 2.13, standard deviation: 6.56, confidence interval: −3.36–7.61) but the difference was not statistically significant (P = 0.214).
As for adverse events, one patient from the atorvastatin group experienced liver enzyme elevations and another patient experienced mild muscle aches for the first 2 days of atorvastatin therapy. Spontaneous resolution was noted for the two patients.
Discussion
Several studies revealed an association between psoriasis and the metabolic syndrome.[15],[16],[17] Statins are well-established to control dyslipidemia, a known component of the metabolic syndrome. As mentioned, they have been found to have immunomodulatory effects, inhibiting antigen presentation, activation and migration of T-cells to the skin and production of pro-inflammatory cytokines, all of which are important in the immunopathogenesis of psoriasis. Thus, they have been investigated as anti-psoriatic treatments, hoping to hit two birds with one stone, treating both psoriasis and its associated metabolic syndrome.
The present study employed the use of atorvastatin 40 mg once a day. Atorvastatin is the most studied class of statin in terms of anti-inflammatory effects and has been shown to have more anti-inflammatory effects compared to the other classes. These anti-inflammatory effects were mainly illustrated by the decrease in C-reactive protein levels.[18],[19],[20],[21] Decrease in C-reactive protein levels was evident starting at a dose of 40 mg with atorvastatin 20 mg not showing any significant decrease in inflammatory markers compared to placebo.[22]
In order to assess the efficacy of atorvastatin in psoriasis, we used PASI-50 and DLQI scores as clinical parameters. Although 75% reduction in PASI scores or PASI-75 has been considered by the US Food and Drug Administration as the benchmark of primary endpoints in assessing therapies for psoriasis, Carlin et al. contested that PASI-50 is already a clinically significant endpoint. Improvement in quality of life already exists at PASI-50, as measured by the Dermatology Life Quality Index. Effective, meaningful therapies are consistently differentiated from placebo at PASI-50 as evidenced by histologic and photographic parameters of other clinical trials. Furthermore, patients achieving PASI-75 frequently defer therapy until they are well below PASI-50.[23] The Dermatology Life and Quality Index, on the other hand, was developed to evaluate the quality of life of patients with skin diseases.[24] It was reported to be highly correlated to clinical endpoints among psoriasis patients at baseline and at week 12 and was the most responsive to changes in endpoints among other indexes.[25]
Our study showed a greater mean reduction in PASI scores in the atorvastatin group compared to the placebo group, although differences were not statistically significant. Using the per-protocol analysis, the atorvastatin group was noted to have significantly higher mean percentage reductions in PASI earlier in the treatment course, although the improvement was not sustained beyond the 3rd month in some patients. Patients from the placebo group were similarly able to achieve PASI-50 at the end of 6 months. This implies that the adjunctive use of atorvastatin to topical corticosteroids in the treatment of mild to moderate plaque type psoriasis apparently did not produce a significant added benefit. This lack of additional benefit was likewise reflected in the change in DLQI scores which was not found to be significantly different between the two groups. Our study also showed that a Class III (mid-potency) topical corticosteroid, betamethasone valerate 0.1% ointment was already sufficient for some patients to achieve PASI-50 at 6 months. However, the differences in the results of the two analyses (intention-to-treat and per protocol) may signify that a high dropout rate might have affected the results with the conclusions being soft.
Our findings are similar to two previous studies: that by Colsman and Sticherling and Faghihi et al.[7],[11] Our protocol is closer to that of Faghihi, where they examined the effect of atorvastatin 40 mg/tablet on patients with mild psoriasis (PASI <12) for 12 weeks.[11] Other studies reporting significant improvement were up to week 8 only,[6],[8],[10] except that of Vasiuk et al.[9] Similarly, we saw significant improvement on month 2 and 3, but afterward, the improvement was comparable in the two groups. We therefore surmise that statins might initially give faster improvement up to month 2, but that these effects would not be sustained.
This study enrolled patients with only mild to moderate psoriasis. This is in contrast to the studies of Vasiuk et al. and Guitan and Paliza which enrolled patients with severe psoriasis with PASI scores ranging from 13.3 to 30.03.[9],[10] Hence, a larger treatment effect must have been observed in the two previous studies as a result of the higher baseline PASI scores. This difference may have resulted in the contrast compared to our findings. We decided not to enroll patients with severe psoriasis due to ethical reasons. Psoriasis treatment guidelines advise the initiation of systemic therapy once a PASI score of 10 is reached and hence it would be unethical to withhold systemic therapy from patients with severe psoriasis for 6 months considering that the placebo group would essentially be using topical corticosteroids only.
Our study also attempted to correlate the decrease in PASI scores with the decrease in lipid profile levels and the decrease in high-sensitivity C-reactive protein. Results showed that despite the decrease in the total cholesterol and low-density lipoprotein-cholesterol levels in the atorvastatin group, PASI scores did not decrease correspondingly. This finding emphasizes that if indeed atorvastatin had anti-inflammatory effects, these effects were independent of its lipid-lowering capabilities. Interestingly, an expected overall decrease in hsCRP levels in the atorvastatin group was not observed in our study. This observation may be because patients might have other inflammatory processes going on in their bodies and atorvastatin 40 mg might not be enough to control these ongoing inflammatory processes; and plausibly because high-sensitivity C-reactive protein is not a specific inflammatory marker for psoriasis. A high-sensitivity C-reactive protein test is a more sensitive measure of C-reactive protein using laser nephelometry and can detect levels as low as 0.04 mg/dL. C-reactive protein is an acute-phase reactant produced by the liver in response to the release of interleukin-6 by macrophages and thus is an indirect measure of interleukin-6, an inflammatory marker involved in the pathogenesis of psoriasis. However, psoriasis has a very complex immunopathogenesis with a myriad of inflammatory cytokines involved (tumor necrosis factor-α, interferon-γ, interleukin-1β, interleukin-6) and a suitable inflammatory marker for research remains to be identified. Although several studies have shown significant correlation of C-reactive protein levels with psoriasis disease severity, especially for moderate to severe psoriasis, there is no sufficient evidence that the same association exists for mild disease.[26]
Atorvastatin, until recently, has been available in the market as a very expensive drug, costing as much as ninety pesos or 1.8 USD for a 40 mg/tablet. It is fortunate that cheaper options are now available, costing as low as 15 pesos or 0.3 USD for a 40 mg/tablet. Nonetheless, this additional cost for the treatment of psoriasis should be weighed against its benefits.
Limitations
The remarkably high dropout rate decreases the robustness of study results. Interpretations of statistical tests should therefore be taken with caution.
Conclusions
There is no sufficient evidence to say that adjunctive use of atorvastatin 40 mg/day for 6 months has a significant added benefit on placebo in the treatment of Filipino adults with mild to moderate plaque type psoriasis. Per-protocol analysis however showed that there is a trend to benefit with atorvastatin 40 mg/day in terms of a slightly faster achievement of PASI-50. Additional cost of this treatment should be weighed against its potential benefits. Nonetheless, atorvastatin would still be helpful for patients who need cholesterol- and low-density lipoprotein-lowering agents (i.e., psoriasis patients with concomitant metabolic syndrome)
Financial support and sponsorship
This study was partially financially supported by the Philippine Dermatological Society.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Gudjonsson JE, Elder JT. Psoriasis. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick's Dermatology in General Medicine. 7th ed. New York, McGraw-Hill; 2008. p. 169-93.
[Google Scholar]
|
| 2. |
Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med 2001;344:1959-65.
[Google Scholar]
|
| 3. |
Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation 1999;100:230-5.
[Google Scholar]
|
| 4. |
Albert MA, Danielson E, Rifai N, Ridker PM; PRINCE Investigators. Effect of statin therapy on C-reactive protein levels: The pravastatin inflammation/CRP evaluation (PRINCE): A randomized trial and cohort study. JAMA 2001;286:64-70.
[Google Scholar]
|
| 5. |
Biasucci LM, Biasillo G, Stefanelli A. Inflammatory markers, cholesterol and statins: Pathophysiological role and clinical importance. Clin Chem Lab Med 2010;48:1685-91.
[Google Scholar]
|
| 6. |
Shirinsky IV, Shirinsky VS. Efficacy of simvastatin in plaque psoriasis: A pilot study. J Am Acad Dermatol 2007;57:529-31.
[Google Scholar]
|
| 7. |
Colsman A, Sticherling M. Simvastatin in psoriasis: Ambiguous effects. Acta Derm Venereol 2010;90:411.
[Google Scholar]
|
| 8. |
Naseri M, Hadipour A, Sepaskhah M, Namazi MR. The remarkable beneficial effect of adding oral simvastatin to topical betamethasone for treatment of psoriasis: A double-blind, randomized, placebo-controlled study. Niger J Med 2010;19:58-61.
[Google Scholar]
|
| 9. |
Vasiuk IU, Perlamutrov IU, Shkol'nik MN, Shkol'nik EL. Possibilities of atorvastatin in complex management of extensive psoriasis in patients with arterial hypertension. Kardiologiia 2010;50:37-46.
[Google Scholar]
|
| 10. |
Guitan GR, Paliza AC. A randomized, double-blind, placebo-controlled trial on the efficacy of simvastatin in the treatment of chronic plaque type psoriasis. J Philipp Dermatol Soc 2012;21:13-22.
[Google Scholar]
|
| 11. |
Faghihi T, Radfar M, Mehrabian Z, Ehsani AH, Rezaei Hemami M. Atorvastatin for the treatment of plaque-type psoriasis. Pharmacotherapy 2011;31:1045-50.
[Google Scholar]
|
| 12. |
Ghazizadeh R, Tosa M, Ghazizadeh M. Clinical improvement in psoriasis with treatment of associated hyperlipidemia. Am J Med Sci 2011;341:394-8.
[Google Scholar]
|
| 13. |
Torres RA, Silva SA, Magalhães RF, Morcillo AM, Velho PE. Comparison of quality of life questionnaires and their correlation with the clinical course of patients with psoriasis. An Bras Dermatol 2011;86:45-9.
[Google Scholar]
|
| 14. |
Finlay A. (Personal communication with the author through e-mail, May 26, 2012).
[Google Scholar]
|
| 15. |
Dreiher J, Weitzman D, Davidovici B, Shapiro J, Cohen AD. Psoriasis and dyslipidaemia: A population-based study. Acta Derm Venereol 2008;88:561-5.
[Google Scholar]
|
| 16. |
Gisondi P, Tessari G, Conti A, Piaserico S, Schianchi S, Peserico A, et al. Prevalence of metabolic syndrome in patients with psoriasis: A hospital-based case-control study. Br J Dermatol 2007;157:68-73.
[Google Scholar]
|
| 17. |
Cohen AD, Sherf M, Vidavsky L, Vardy DA, Shapiro J, Meyerovitch J. Association between psoriasis and the metabolic syndrome. A cross-sectional study. Dermatology 2008;216:152-5.
[Google Scholar]
|
| 18. |
Patel TN, Shishehbor MH, Bhatt DL. A review of high-dose statin therapy: Targeting cholesterol and inflammation in atherosclerosis. Eur Heart J 2007;28:664-72.
[Google Scholar]
|
| 19. |
Macin SM, Perna ER, Farías EF, Franciosi V, Cialzeta JR, Brizuela M, et al. Atorvastatin has an important acute anti-inflammatory effect in patients with acute coronary syndrome: Results of a randomized, double-blind, placebo-controlled study. Am Heart J 2005;149:451-7.
[Google Scholar]
|
| 20. |
Ascer E, Bertolami MC, Venturinelli ML, Buccheri V, Souza J, Nicolau JC, et al. Atorvastatin reduces proinflammatory markers in hypercholesterolemic patients. Atherosclerosis 2004;177:161-6.
[Google Scholar]
|
| 21. |
McCarey DW, McInnes IB, Madhok R, Hampson R, Scherbakov O, Ford I, et al. Trial of atorvastatin in rheumatoid arthritis (TARA): Double-blind, randomised placebo-controlled trial. Lancet 2004;363:2015-21.
[Google Scholar]
|
| 22. |
Lewandowski M, Kornacewicz-Jach Z, Millo B, Zielonka J, Czechowska M, Kaliszczak R, et al. The influence of low dose atorvastatin on inflammatory marker levels in patients with acute coronary syndrome and its potential clinical value. Cardiol J 2008;15:357-64.
[Google Scholar]
|
| 23. |
Carlin CS, Feldman SR, Krueger JG, Menter A, Krueger GG. A 50% reduction in the psoriasis area and severity index (PASI 50) is a clinically significant endpoint in the assessment of psoriasis. J Am Acad Dermatol 2004;50:859-66.
[Google Scholar]
|
| 24. |
Finlay AY, Khan GK. Dermatology life quality index (DLQI) – A simple practical measure for routine clinical use. Clin Exp Dermatol 1994;19:210-6.25.
[Google Scholar]
|
| 25. |
Shikiar R, Willian MK, Okun MM, Thompson CS, Revicki DA. The validity and responsiveness of three quality of life measures in the assessment of psoriasis patients: Results of a phase II study. Health Qual Life Outcomes 2006;4:71.
[Google Scholar]
|
| 26. |
Beygi S, Lajevardi V, Abedini R. C-reactive protein in psoriasis: A review of the literature. J Eur Acad Dermatol Venereol 2014;28:700-11.
[Google Scholar]
|
Fulltext Views
2,802
PDF downloads
1,558





