Translate this page into:
Autophagy: A brief overview in perspective of dermatology
Corresponding Author:
Rahul Nagar
Department of Dermatology, Venereology and Leprosy, Second Floor, New OPD Building, Mahatma Gandhi Memorial Medical College and Maharaja Yashwantrao Hospital, Indore - 452 001, Madhya Pradesh
India
rahulnagar.doc@gmail.com
| How to cite this article: Nagar R. Autophagy: A brief overview in perspective of dermatology. Indian J Dermatol Venereol Leprol 2017;83:290-297 |
Abstract
Autophagy, literally meaning “self-eating,” is an intracellular catabolic process of delivering cytosol and/or its specific content to the lysosomes for degradation.The resulting macromolecular constituents are recycled and utilized again by the cells. Basal level autophagy plays an important role in cellular homeostasis through the elimination of the old or damaged organelles, as well as aggregated intracellular proteins. Autophagy refers to sequestration of intact organelles along with a portion of cytosol, into a double-or multi-membrane structure known as phagophore, which elongates, and after closure, forms a vesicular structure known as the autophagosome. Subsequently, the mature autophagosome fuses with a lysosome, thereby forming a single membrane structure, an autolysosome. Autophagy plays a critical role in inflammation, autoimmunity and cellular differentiation. Skin serves as the first line of defense against a variety of environmental insults and autophagy is thought to be a form of an endogenous defense mechanism against such environmental derangements. Autophagy has been linked with keratinocyte differentiation and melanocyte survival, as well as with the pathogenesis of diverse skin disorders including systemic lupus erythematosus, systemic sclerosis, psoriasis, vitiligo, infectious skin diseases and cancer. Autophagy has been one of the most studied phenomena in cell biology and pathophysiology, and given its broad clinical implications, has become a major target for drug discovery. The last decade has seen a substantial upsurge in autophagy-related research and publications; still, the dermatology literature appears to be less initiated. Autophagy will probably change our understanding of dermatological disorders/medicines. Hence, a basic knowledge of autophagy is a prerequisite to understand the developments in the field of autophagy-related research.Introduction
Autophagy, literally meaning “self-eating,” is an intracellular catabolic process of delivering cytosol and/or its specific content to the lysosomes for degradation. The resulting macromolecular constituents are then recycled and utilized by the cells.[1] The term autophagy was proposed by Christian de Duve in 1963 at a CIBA Foundation symposium on lysosomes.[2] Basal level autophagy plays an important role in cellular homeostasis through elimination of the old or damaged organelles, as well as aggregated intracellular proteins.[2] On the other hand, during conditions of cellular stress, such as nutrient deprivation/starvation, hypoxia, oxidative stress, pathogen infection, radiation or anticancer drug treatment, the level of autophagy is augmented, resulting in adaptation and cell survival (cytoprotective response).[1] In recent years, studies have shown that autophagy plays a critical role in inflammation, pathogen clearance and antigen presentation.[3] Consequently, dysregulated autophagy has been implicated in the pathogenesis of diverse human disorders, for example, infectious diseases, Crohn's disease, neurodegenerative disorders, autoimmune disease, metabolic disorder, cancer and also in cell growth and death.[4]
Autophagy has been reported to play a role in terminal epidermal keratinization and in hair growth during early stages of differentiation.[5] Importantly, autophagy pathways have a place in fine tuning the inflammation response in keratinocytes; a reduction in keratinocyte autophagy has been shown to be associated with the development of inflammatory response, such as in psoriasis.[6] Furthermore, autophagy has a substantial role in the degradation of melanosomes in epidermal keratinocytes, and in melanocyte survival and proliferation. Hence, it could influence the development of basal skin color, as well as the development of diseases affecting melanocytes.[7],[8] In addition, there is reduction in autophagy with age, leading to accumulation of damaged proteins and organelles, and increase in autophagy has been shown to increase the longevity of certain experimental organisms.[9]
Owing to the pathophysiological significance of autophagy, it has been the subject of intensive studies, particularly during last decade. Due to the tremendous amount of experimental work that has been performed and that has been going on, it is nearly impossible to be completely inclusive. This review will focus on the mammalian autophagy process and its major signaling regulators.
Types of Autophagy
In mammalian cells, at least three primary types of autophagy exist: Macroautophagy, microautophagy and chaperone-mediated autophagy [Figure - 1].[10] Macroautophagy refers to sequestration of intact organelles along with a portion of cytosol, into a double-or multi-membrane structure known as phagophore which elongates and after closure forms a vesicular structure, the autophagosome. Subsequently, the mature autophagosome fuses with the lysosome, thereby forming a single membrane structure, autolysosome. This transit is mediated along the microtubules.[1] Micro-autophagy involves direct engulfment of cytoplasm at the lysosomal surface, while in chaperone-mediated autophagy soluble unfolded proteins are directly translocated across the lysosomal membrane.[10]
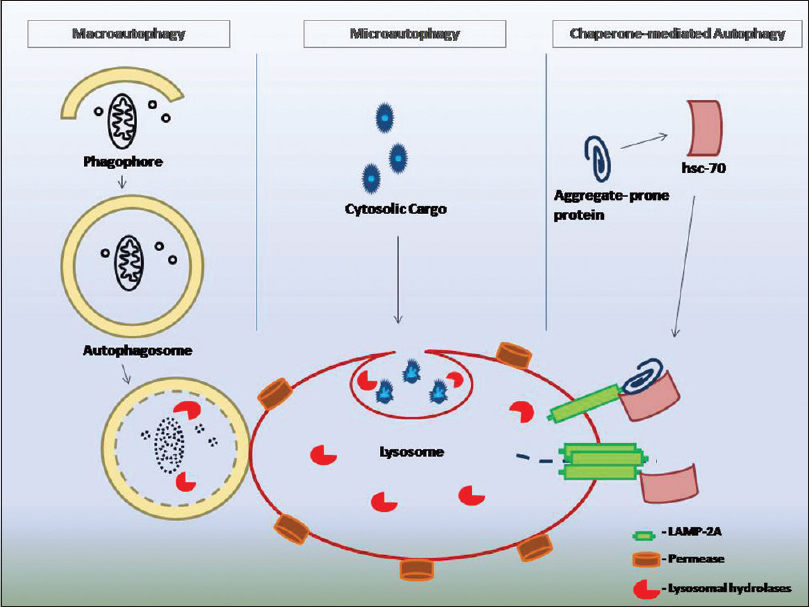 |
| Figure 1: Schematic depiction of three types of autophagy |
Micro-and macro-autophagy can be selective or nonselective.[2] Nonselective autophagy is turnover of bulk cytoplasm, whereas selective autophagy implies sequestration of specific targets, for example, damaged or superfluous organelles, as well as microbes.[2] The sequestration of specific substrates is given specific names: Mitochondrial sequestration is termed as mitophagy, endoplasmic reticulum reticulophagy, lipid droplets lipophagy, peroxisomes pexophagy, ribosomes ribophagy, cytoplasmic aggregates aggregaphagy and invading pathogens xenophagy. Furthermore, endosomes, lysosomes, secretory granules and inflammatory proteins can also be a target of selective autophagy.[11]
Ultimately, all the three types of autophagy depend on lysosomal function, failure of which carries pathological consequences. This review will focus on a brief overview of macroautophagy, and unless otherwise specified, hereafter “autophagy” refers to macroautophagy.
Molecular Machinery of Autophagy
The process of autophagy can be divided into these main steps: Initiation, vesicle nucleation, membrane elongation, closure, maturation and degradation [Figure - 2].[12] More than thirty autophagy-related genes (ATG genes) and their proteins are known, but one subset consisting of approximately 18 genes is shared among various types of autophagy. The corresponding gene products of this subset are required for autophagosome formation and are termed the “core” molecular machinery. These core autophagy-related proteins are composed of four subgroups [Figure - 2]: (a) The unc-51-like kinase complex, (b) Beclin1 complex - the class III phosphatidylinositol 3-kinase complex, (c) two ubiquitin-like proteins, Atg12 and light chain 3, and (d) transmembrane proteins mammalian Atg9 and its cycling system.[10]
 |
| Figure 2: Schematic model of autophagy. The class III phosphatidylinositol 3-kinase complex activation occurs downstream to the unc-51-like kinase complex. The class III phosphatidylinositol 3-kinase complex recruits autophagy-related proteins at the phagophore assembly site and initiates phagophore formation. Phagophore elongates to forms a vesicular structure, autophagosome. Mature autophagosome fuses with lysosome, thereby forming single membrane structure, autolysosome |
Initiation of autophagy requires two protein kinase complexes:First, the unc-51-like kinase complex along with associated mammalian target of rapamycin complex 1 and second, the Beclin1-vps34-p150-Atg14L complex along with associated antiapoptotic Bcl 2 protein.
- The unc-51-like kinase complex, an initial complex that regulates the induction of autophagosome formation. The unc-51-like kinase complex consists of a serine/threonine kinase, unc-51-like kinase 1 and 2, Atg13 and a scaffold protein FIP200. The ULK1/2-Atg13-FIP200 complex binds with mammalian target of rapamycin complex 1 in nutrient rich conditions. An Atg101 is also found to interact with unc-51-like kinase 1. The mammalian target of rapamycin complex 1 (mTORC1) is a complex, consisting mammalian target of rapamycin (mTOR) kinase, GβL, PRAS 40 and raptor (regulatory-associated protein of mTOR).[13]
- The class III phosphatidylinositol 3-kinase complex (Beclin1-vps34-p150-Atg14L) acts at the stage of vesicle nucleation and in the recruitment of phosphatidylinositol 3-phosphate-binding proteins to nucleation site. Formation of class III complex includes vacuolar protein sorting 34(a phosphatidylinositol 3-kinase), Beclin 1 and p150. An Atg14-like protein (Atg14L or Barkor) and ultraviolet irradiation resistant-associated gene are also associated with this complex. Ultraviolet irradiation resistant-associated gene activates and Bcl2 family proteins inhibit autophagy through theirinteraction with Beclin 1.[14],[15]
Dissociation of mTORC1 from unc-51-like kinase complex and dissociation of Bcl2 from Beclin1 are required for autophagy induction.[1] Under stressful conditions, AMP-activated protein kinase (AMPK) induces autophagy by activating unc-51-like kinase complex and by inhibiting mTORC1.[10] These events lead to subsequent recruitment and activation of the Beclin1 complex at the membrane, inducing nascent phagophore formation.[10] The vacuolar protein sorting-34 marks the site where phagophore emerges from the endoplasmic reticulum by generating a phosphatidylinositol-3-phosphate rich structure called omegasome.[12] Phosphatidylinositol-3-phosphate accumulation is necessary for recruitment of the phagophore elongation complex Atg12-Atg5-Atg16L.[12]
- Two ubiquitin-like proteins such asAtg12 and light chain 3 (microtubule-associated protein 1 light chain 3) and their conjugation systems function during elongation and expansion of the phagophore membrane. Light chain 3 conjugation system is also required for closure of phagophore.[1],[2] The Atg12 is conjugated to Atg5 (to form Atg12-Atg5 complex) in the presence of Atg7 and Atg10 [Figure - 1]. The Atg12-Atg5 complex interacts with Atg16L which oligomerizes to form a large multimeric complex called Atg16L complex. Formation of Atg16L complex promotes conjugation of another important protein, the light chain 3. The soluble light chain 3 is first cleaved by Atg4 to generate light chain 3-I which is conjugated to phosphatidylethanolamine (lipidation of light chain 3) to generate light chain 3-II through the help of Atg7 and Atg3. Thus, the generated light chain 3-II (lapidated form of light chain 3) is attached to both faces of the phagophore membrane.[1],[16] The light chain 3-II has the ability to determine membrane curvature, thus has a role in regulating the size of autophagosome.[17] Several cytosolic organelles donate to the phagophore membrane during membrane elongation process. It is not completely clear by what mechanism the additional membranes are delivered to and fused with the growing phagophore.[17] However, it has been observed that Atg5-Atg12-Atg16L-complex mediated fusion, thereby increasing the size of the membrane constituting the phagophore.[18] This process also serves as a prerequisite for optimal acquisition of light chain 3 and thus, for progression from autophagosome precursor to phagophore.[18]
- Two transmembrane proteins, mammalian Atg9 and vacuole membrane protein 1, play a role in membrane delivery to the expanding phagophore; Atg9 is located in trans-Golgi network and in late endosomes and is redistributed to peripheral sites upon initiation of autophagy.[1] It has been proposed that Atg9 contributes to delivery of membrane to the constructing autophagosome.[19] Whereas, the other transmembrane protein, the vacuole membrane protein 1 recruits vesicle nucleation complexes at the phagophore.[19]
In mammalian cells, the autophagosome-lysosome fusion requires soluble N-ethylmaleimide-sensitive factor attachment protein receptor and Rab proteins.[20] Rab proteins are small GTPases involved in autophagosome biogenesis and in autophagosome maturation.[21] Soluble N-ethylmaleimide-sensitive factor attachment protein receptor proteins mediate transport along actin filaments or microtubules and the docking and fusion of vesicles with their proper target organelle.[22] Histone deacetylase 6, a microtubule-associated deacetylase, promote autophagy by targeting protein aggregates to dynein motor for transport to lysosome.[22] After fusion, degradation of the inner vesicle is dependent on a series of lysosomal acid hydrolases, including proteinases, lipases and cathepsins. The inner vesicle is degraded along with membrane and attached light chain 3-II, resulting in single membrane autolysosome. The resulting small molecules from the degradation are transported back to the cytosol for protein synthesis and maintenance of cellular functions. The permeases represent the last step in the degradation and recycling process by functioning as effluxers of small molecules. Interestingly, the mTOR is reactivated upon autophagy termination by amino acid release and stimulates extrusion of lysosomal-derived membranes from autolysosomes to form new functional lysosomes.[2],[12],[23] Cellular and foreign material destined for degradation reach lysosomes through autophagy or endocytosis or phagocytosis or direct transport. Lysosomes are involved in the degradation of a wide variety of substances into their basic building blocks such as proteins, glycosaminoglycans, sphingolipids, glycogen, nucleic acid, oligosaccharides and lipids. Notably, lysosomal storage disorders are recognized as a cohort of nearly sixty different inherited disorders with each sharing a genetic defect that renders the lysosomal system dysfunctional, and consequently, failure to degrade and recycle the sequestered materials in lysosomes. In most cases, there is an impairment of autophagic flux causing a secondary accumulation of autophagy substrates, dysfunctional mitochondria and factors involved in autophagosome formation. Accordingly, authors have suggested lysosomal storage disorders as “autophagy disorders.”[24],[25]
The chaperone-mediated autophagy functions in a different manner from macroautophagy. Chaperone-mediated autophagy utilizes a cytosolic chaperone, heat shock-cognate protein of 70 KDa and the lysosomal-associated membrane protein 2A receptor. The cytosolic heat shock-cognate protein of 70 KDa recognizes a specific pentapeptide sequence Lys-Phe-Gln-Arg-Gln, (also known as KFERQ sequence) of the substrate protein, leading to the substrate-heat shock-cognate protein of 70 KDa complex to target the lysosomal surface, where it binds to the lysosomal-associated membrane protein 2A.[26] The lysosomal-associated membrane protein 2A multimerizes at the lysosomal surface and thus internalizes the substrate.[26] Here, the Lys-heat shock-cognate protein of 70 KDa (lysosome-resident heat shock-cognate protein of 70 KDa) and the lysosomal heat shock protein 90 also have shown to play a critical role in assembly and disassembly of lysosomal-associated membrane protein 2A on the lysosomal surface.[26] Basal chaperone-mediated autophagy activity has been described in multiple cell types as a control system to maintain functionality of proteome. Macroautophagy is the first line of defense during cellular stress; however, when the stress persists beyond 6–8 h, the induction of chaperone-mediated autophagy ensures cellular homeostasis through selective removal of damaged proteins.[26]
Signaling Pathways Regulating Macroautophagy
In conditions of cellular stress, such as nutrient deprivation/starvation, hypoxia, oxidative stress, pathogen infection, radiation and pharmacological agents, the level of autophagy is augmented.[1] Autophagy is promoted through two well-characterized signaling cascades referred to as (a) the mTOR dependent and (b) mTOR independent pathways.
- The mTOR-dependent pathway utilized during autophagy is induced by starvation, growth factors/insulin and low energy; here, the tuberous sclerosis complex 1 and 2 plays an important role. When conjugated with each other, it converts Rheb protein bound GTP to GDP. The Rheb protein in its GTP-bound form activates mTORC1 and suppresses autophagy, whereas its conversion to GDP-Rheb induces autophagy; insulin activates mTOR by impeding tuberous sclerosis complex 1-2 complex formation. Low-energy levels are sensed by an AMP-activated protein kinase which facilitates tuberous sclerosis complex 1-2 complex formation, thus inhibition of mTOR and finally leading to activation of autophagy. Cytosolic amino acid levels are sensed by Rag proteins which directly activates mTOR.[27] The p53 tumor suppressor manages genotoxic stress or oncogenic activation. The activated p53 induces autophagy through AMP-activated protein kinase-tuberous sclerosis complex 1/2 pathway.[28]
- The mTOR-independent pathway is regulated by intracellular Ca ++ levels or by G-protein-coupled receptor. Calcium enters the cell through L-type Ca ++ channels and activates calpain which inhibits autophagy by the removal of Atg5 protein. Activated G-protein-coupled receptor generates inositol triphosphate which inhibits autophagy through the formation of Beclin1-Bcl2 complex. The G-protein-coupled receptor could be activated either by its legend extracellularly or by calpain intracellularly.[27]
Methods to Measure Autophagy
The most traditional method to measure autophagy utilizes electron microscopy, by which the autophagosome can be visualized easily.[28] However, more readily available fluorescence microscopy and biochemical methods have become popular detection tools.[27] Light chain 3-II is consistently associated with both the surfaces of autophagosomes and thus, it is a useful indicator of autophagosome initiation.[29] The light chain 3-II can be measured through Western Blot technique. Similarly, immunostaining of light chain 3 in fixed tissue or cell can help measuring through high-throughput fluorescence microscopy.[30] Western blot analysis is only a snapshot of a dynamic process; instead measurement of autophagy flux in the presence or absence of lysosomal blockade provides essential information.[30] Autophagic flux refers to the rate of transit of autophagosome cargo through lysosomal degradation.[30]
In one of the in vivo methods of measuring autophagy flux, tissue from mice subjected to lysosomal blockade and untreated comparators is harvested at a specified time (usually 2–4 h) after blockade. Differential accumulation of autophagosomes can be assessed by electron- or fluorescent-microscopy or by light chain 3-II Western blot. Cryosections are used for the measurement of fluorescence; in case of autophagy induction, the fluorescent puncta substantially increases, although a small number of fluorescent puncta are observed even under normal conditions owing to a basal level autophagy. Thus, the effect of autophagy inducers/inhibitors can be monitored by investigating the pattern of fluorescent puncta.[31]
Pharmacological Agents That Target Autophagy Machinery
Pharmacological agents that activate and inhibit autophagy have been used in research settings, and their potential application in developing new treatments for human diseases are enumerated in [Table - 1].[20],[25],[26],[27],[32],[33],[34] However, most compounds discussed here affect the regulatory mechanism of autophagy and knowledge of molecules that can directly target autophagy is meager. The development of modulators of autophagy is still in its early phase, and has not been thoroughly investigated in humans. Even for those Food and Drug Administration-approved drugs that have been shown in experimental systems to modulate autophagy, it remains to be seen whether the concentration required for modulating autophagy in vivo can be safely achieved.

Autophagy in Autoimmune Skin Disorders
A recent study has linked several single nucleotide polymorphisms in the ATG16L1 gene with susceptibility to development of psoriasis. Defects in autophagy lead to inflammation and keratinocyte proliferation, the two pathological characteristics of psoriasis. Moreover, ATG16L1 gene product plays an important role in bacterial handling and antigen presentation through processes mediated by autophagy machinery; of note here is triggering or exacerbation of psoriasis following bacterial infection.[3] Toll-like receptors 2 which are key pattern recognition molecules in innate immunity are up-regulated. A link between autophagy inhibition and dysregulated innateimmune response have been suggested in psoriasis.[3] In support of this concept, many first-line agents in the treatment of psoriasis such as vitamin D analogs, retinoids, sirolimus and ultraviolet B therapy can induce autophagy, though these drugs might provide clinical benefits independent of autophagy activation.[3]
It has been shown that autophagy is increased in lupus B-and T-lymphocytes. Genetic variations in ATG5 (involved in innate immunity against environmental stress like ultraviolet light) have been found in patients of systemic lupus erythematosus.[3] Autophagy activators amiodarone, carbamazepine, chlorpromazine, clonidine, lithium, minocycline, valproic acid and verapamil have been associated with drug-induced lupus; whereas known autophagy inhibitors, chloroquine and hydroxychloroquine are used to treat systemic lupus erythematosus.[34] A small study had assessed the autophagy activity and the autophagosome formation by immunofluorescence and electron microscopy, respectively, both in patients with systemic sclerosis and in healthy controls. They had found autophagy activity in only the former, hence suggesting a role of autophagy in the pathogenesis of this condition.[35]
A cohort study from Korea has linked nonsegmental vitiligo with ultraviolet irradiation resistant-associated gene polymorphisms.[36] Recent reports suggest the involvement of intramelanocytic oxidative stress in the pathogenesis of vitiligo, whereas autophagy has been involved in melanocytic redox homeostasis.[30],[37] The melanin levels in human skin samples cultured ex vivo and in human skin substitutes in vitro were substantially diminished by activators of autophagy and increased by its inhibitors.[7] This data suggests a plausible link between the pathogenesis of vitiligo and autophagy.
Autophagy in Infectious Skin Diseases and Inflammation
Nakagawa et al. provided the first evidence that autophagy is important in defense against bacterial pathogens that invade the cytosol.[38] Intracellular bacteria such as Mycobacterium tuberculosis, Salmonella, Listeria, Shigella, and group A Streptococcus are optimally degraded through macroautophagy.[39] Curiously macroautophagy can also participate in the elimination of strictly extracellular microorganisms like yeast or Escherichia coli. In this case, a process called light chain 3-associated phagocytosis leads to phagosome decoration by light chain 3-II protein.[39] Xenophagy and light chain 3-associated phagocytosis are useful terms to describe some aspects of a continuum of autophagic machinery engagement with invading microbes.[40] Xenophagy implies engulfment of cytosolic microbes into autophagosomes, whereas light chain 3-associated phagocytosis is a process that engages parts of autophagic machinery when an extracellular cargo is engulfed by phagocytosis.[40] In most cases, autophagic response leading up to xenophagy or light chain 3-associated phagocytosis are guided by several types of pattern recognition receptors and further modulated by cytokines and cellular immune network.[41] Cytokines modulate autophagy by binding to their specific receptors located at the cytoplasmic membrane. In a general way, Th1 cytokines (interlekuin-2, tumor necrosis factor-α and interferon-γ) are considered as autophagy inducers, whereas Th2 cytokines (interlekuin-4,-5,-6,-10 and-13) are regarded as autophagy repressors.[41] At the cellular level, the presence of pathogens is detected by pattern recognition receptors located at plasma membrane (toll-like receptors) or at endosomal membrane (toll-like receptor 3, toll-like receptor 7, toll-like receptor 8 and toll-like receptor 9) or in the cytosol (nucleotide oligomerization domain-like receptors; retinoic acid-inducible gene-I-like receptors; C-type lectin-like receptors).[7] These innate immune receptors recognize highly conserved structural motifs present on microbes, termed pathogen-associated molecular patterns. They also detect a danger-associated molecular pattern that signalizes host cellular damage.[12] Autophagy influences development, repertoire selection, maturation, homeostasis, function and polarization of T cells. Moreover, autophagy is necessary for the maintenance of memory B cells, where autophagy-dependent endoplasmic reticulum maintenance in plasma cells is necessary to balance immunoglobulin secretion and loss of autophagy results in abnormal hypersecretion of immunoglobulins. Autophagy is also important for survival and homeostasis of the bone marrow plasma cell pool and long-lasting humoral immunity. Thus, autophagy regulates both innate and adaptive immunity.[40]
Notably, methicillin-resistant Staphylococcus aureus strains have shown a significant resistance to autophagic degradation; S. aureus was shown to replicate within autophagosomes and subsequently escape into the cytoplasm.[42] In S. aureus containing phagosomes, these compartments are not acidified or acquire the lysosomal marker lysosomal-associated membrane protein-2, resulting in an arrest of autophagosome maturation and lack of fusion with lysosomes. Recent studies have shown that autophagy plays an important role in controlling the spread of fungal infection and susceptibility to disease. Consistent with these findings, genetic disruption ofAtg5 in murine macrophages resulted in decreased Candida albicans uptake and infection.[43]
A study had suggested an important role for autophagy in the defense of keratinocytes against human papillomavirus 16.[44] This study showed that human papillomavirus 16 infectivity is dramatically enhanced by knockdown of essential autophagy genes, as well as by biochemical inhibition of autophagy. Herpes simplex virus-1, influenza virus, human immunodeficiency virus, hepatitis C virus and coronavirus are able to induce autophagy; however at the same time, these viruses have been shown to inhibit the last step of autophagosome-lysosome fusion which promotes viral replication and reduces cell apoptosis.[45] Interestingly, a significant increase in autophagy has been detected in the peripheral blood mononuclear cells of patients who remain asymptomatic for more than 10 years because they are able to control human immunodeficiency virus viremia.[46]
The capacity of M.tuberculosis to survive and replicate in host macrophages is central to its pathogenesis. One of the mechanisms behind the survival of thebacterium is its ability to block phagocytosis and thus avoid killing. Some populations of tuberculosis patients are prone to develop tubercular infection owing to polymorphisms in genes linked to autophagy pathways. Interestingly, mycobacterium killing can be restored through exogenous induction of autophagy in infected macrophages.[46]
Autophagy in Skin Cancer
In general, autophagy acts as a tumor suppressor in normal cells but serves as a survival mechanism for established tumors.[47] Squamous cell carcinoma and melanoma have shown high levels of autophagy, in which high autophagic activity is associated with tumor aggressiveness. A recent report indicates that inhibition of autophagy by chloroquine could enhance cell death induced by the flavonoid luteolin in metastatic squamous cell carcinoma cells.[48] These data support the view that autophagy upregulation serves as a cytoprotective mechanism in squamous cell carcinoma. In line with this, melanomas with high levels of autophagy were more likely to resist chemotherapy and autophagy was suggested to be a potential therapeutic target.
Concluding Remarks
Autophagy has a significant impact on health, as its important role in the regulation of inflammation, and in mitigating or exacerbating various diseases, is being increasingly recognized. Autophagy has been claimed as one of the most studied phenomena in cell biology and pathophysiology and has become a major target for drug discovery.[49] The last decade has seen a substantial upsurge in autophagy-related research and publications; still, the dermatology literature appears to be less initiated. However, given its broad clinical implications, autophagy will percolate dermatology literature and probably will change our understanding of dermatological disorders/medicines. Hence, a basic knowledge of autophagy is a prerequisite to understand the developments in the field of autophagy-related research.
Acknowledgment
The author would like to thank Dr. Subodh Chaturvedi for critical reading and comments on the manuscript.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. | Yang Z, Klionsky DJ. Mammalian autophagy: Core molecular machinery and signaling regulation. CurrOpin Cell Biol 2010;22:124-31. [Google Scholar] |
| 2. | Feng Y, He D, Yao Z, Klionsky DJ. The machinery of macroautophagy. Cell Res 2014;24:24-41. [Google Scholar] |
| 3. | Yu T, Zuber J, Li J. Targeting autophagy in skin diseases. J Mol Med (Berl) 2015;93:31-8. [Google Scholar] |
| 4. | Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature 2008;451:1069-75. [Google Scholar] |
| 5. | Yoshihara N, Ueno T, Takagi A, Oliva Trejo JA, Haruna K, Suga Y, et al. The significant role of autophagy in the granular layer in normal skin differentiation and hair growth. Arch Dermatol Res 2015;307:159-69. [Google Scholar] |
| 6. | Lee HM, Shin DM, Yuk JM, Shi G, Choi DK, Lee SH, et al. Autophagy negatively regulates keratinocyte inflammatory responses via scaffolding protein p62/SQSTM1. J Immunol 2011;186:1248-58. [Google Scholar] |
| 7. | Murase D, Hachiya A, Takano K, Hicks R, Visscher MO, Kitahara T, et al. Autophagy has a significant role in determining skin color by regulating melanosome degradation in keratinocytes. J Invest Dermatol 2013;133:2416-24. [Google Scholar] |
| 8. | Zhang CF, Gruber F, Ni C, Mildner M, Koenig U, Karner S, et al. Suppression of autophagy dysregulates the antioxidant response and causes premature senescence of melanocytes. J Invest Dermatol 2015;135:1348-57. [Google Scholar] |
| 9. | Rubinsztein DC, Mariñ o G, Kroemer G. Autophagy and aging. Cell 2011;146:682-95. [Google Scholar] |
| 10. | Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol 2013;15:713-20. [Google Scholar] |
| 11. | Stolz A, Ernst A, Dikic I. Cargo recognition and trafficking in selective autophagy. Nat Cell Biol 2014;16:495-501. [Google Scholar] |
| 12. | Lapaquette P, Guzzo J, Bretillon L, Bringer MA. Cellular and molecular connections between autophagy and inflammation. Mediators Inflamm 2015;2015:398483. [Google Scholar] |
| 13. | Shang L, Wang X. AMPK and mTOR coordinate the regulation of Ulk1 and mammalian autophagy initiation. Autophagy 2011;7:924-6. [Google Scholar] |
| 14. | Itakura E, Kishi C, Inoue K, Mizushima N. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. MolBiol Cell 2008;19:5360-72. [Google Scholar] |
| 15. | He C, Levine B. The Beclin 1 interactome. CurrOpin Cell Biol 2010;22:140-9. [Google Scholar] |
| 16. | Fujita N, Itoh T, Omori H, Fukuda M, Noda T, Yoshimori T. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. MolBiol Cell 2008;19:2092-100. [Google Scholar] |
| 17. | Juhasz G, Neufeld TP. Autophagy: A forty-year search for a missing membrane source. PLoSBiol 2006;4:e36. [Google Scholar] |
| 18. | Moreau K, Ravikumar B, Renna M, Puri C, Rubinsztein DC. Autophagosome precursor maturation requires homotypic fusion. Cell 2011;146:303-17. [Google Scholar] |
| 19. | Yamamoto H, Kakuta S, Watanabe TM, Kitamura A, Sekito T, Kondo-Kakuta C, et al. Atg9 vesicles are an important membrane source during early steps of autophagosome formation. J Cell Biol 2012;198:219-33. [Google Scholar] |
| 20. | Amaya C, Fader CM, Colombo MI. Autophagy and proteins involved in vesicular trafficking. FEBS Lett 2015;589:3343-53. [Google Scholar] |
| 21. | Bento CF, Puri C, Moreau K, Rubinsztein DC. The role of membrane-trafficking small GTPases in the regulation of autophagy. J Cell Sci 2013;126(Pt 5):1059-69. [Google Scholar] |
| 22. | Iwata A, Riley BE, Johnston JA, Kopito RR. HDAC6 and microtubules are required for autophagic degradation of aggregated huntingtin. J Biol Chem 2005;280:40282-92. [Google Scholar] |
| 23. | Yu L, McPhee CK, Zheng L, Mardones GA, Rong Y, Peng J, et al. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature 2010;465:942-6. [Google Scholar] |
| 24. | Lieberman AP, Puertollano R, Raben N, Slaugenhaupt S, Walkley SU, Ballabio A. Autophagy in lysosomal storage disorders. Autophagy 2012;8:719-30. [Google Scholar] |
| 25. | Settembre C, Fraldi A, Rubinsztein DC, Ballabio A. Lysosomal storage diseases as disorders of autophagy. Autophagy 2008;4:113-4. [Google Scholar] |
| 26. | Kaushik S, Cuervo AM. Chaperone-mediated autophagy: A unique way to enter the lysosome world. Trends Cell Biol 2012;22:407-17. [Google Scholar] |
| 27. | Baek KH, Park J, Shin I. Autophagy-regulating small molecules and their therapeutic applications. Chem Soc Rev 2012;41:3245-63. [Google Scholar] |
| 28. | Feng Z, Zhang H, Levine AJ, Jin S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci U S A 2005;102:8204-9. [Google Scholar] |
| 29. | Eskelinen EL. Maturation of autophagic vacuoles in mammalian cells. Autophagy 2005;1:1-10. [Google Scholar] |
| 30. | Gottlieb RA, Andres AM, Sin J, Taylor DP. Untangling autophagy measurements: All fluxed up. Circ Res 2015;116:504-14. [Google Scholar] |
| 31. | Tanida I, Minematsu-Ikeguchi N, Ueno T, Kominami E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy 2005;1:84-91. [Google Scholar] |
| 32. | Vakifahmetoglu-Norberg H, Xia HG, Yuan J. Pharmacologic agents targeting autophagy. J Clin Invest 2015;125:5-13. [Google Scholar] |
| 33. | Rubinsztein DC, Codogno P, Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov 2012;11:709-30. [Google Scholar] |
| 34. | Gros F, Muller S. Pharmacological regulators of autophagy and their link with modulators of lupus disease. Br J Pharmacol 2014;171:4337-59. [Google Scholar] |
| 35. | Frech T, De Domenico I, Murtaugh MA, Revelo MP, Li DY, Sawitzke AD, et al. Autophagy is a key feature in the pathogenesis of systemic sclerosis. RheumatolInt 2014;34:435-9. [Google Scholar] |
| 36. | Jeong TJ, Shin MK, Uhm YK, Kim HJ, Chung JH, Lee MH. Association of UVRAG polymorphisms with susceptibility to non-segmental vitiligo in a Korean sample. ExpDermatol 2010;19:e323-5. [Google Scholar] |
| 37. | Schallreuter KU, Kothari S, Chavan B, Spencer JD. Regulation of melanogenesis – Controversies and new concepts. ExpDermatol 2008;17:395-404. [Google Scholar] |
| 38. | Nakagawa I, Amano A, Mizushima N, Yamamoto A, Yamaguchi H, Kamimoto T, et al. Autophagy defends cells against invading group A Streptococcus. Science 2004;306:1037-40. [Google Scholar] |
| 39. | Huang J, Brumell JH. Bacteria-autophagy interplay: A battle for survival. Nat Rev Microbiol 2014;12:101-14. [Google Scholar] |
| 40. | Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature 2011;469:323-35. [Google Scholar] |
| 41. | Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nat Rev Immunol 2013;13:722-37. [Google Scholar] |
| 42. | Lenz HD, Haller E, Melzer E, Kober K, Wurster K, Stahl M, et al. Autophagy differentially controls plant basal immunity to biotrophic and necrotrophic pathogens. Plant J 2011;66:818-30. [Google Scholar] |
| 43. | Nicola AM, Albuquerque P, Martinez LR, Dal-Rosso RA, Saylor C, De Jesus M, et al. Macrophage autophagy in immunity to Cryptococcus neoformans and Candida albicans. Infect Immun 2012;80:3065-76. [Google Scholar] |
| 44. | Griffin LM, Cicchini L, Pyeon D. Human papillomavirus infection is inhibited by host autophagy in primary human keratinocytes. Virology 2013;437:12-9. [Google Scholar] |
| 45. | Rey-Jurado E, Riedel CA, González PA, Bueno SM, Kalergis AM. Contribution of autophagy to antiviral immunity. FEBS Lett 2015;589:3461-70. [Google Scholar] |
| 46. | Espert L, Beaumelle B, Vergne I. Autophagy in Mycobacterium tuberculosis and HIV infections. Front Cell Infect Microbiol 2015;5:49. [Google Scholar] |
| 47. | White E, DiPaola RS. The double-edged sword of autophagy modulation in cancer. Clin Cancer Res 2009;15:5308-16. [Google Scholar] |
| 48. | Verschooten L, Barrette K, Van Kelst S, Rubio Romero N, Proby C, De Vos R, et al. Autophagy inhibitor chloroquine enhanced the cell death inducing effect of the flavonoid luteolin in metastatic squamous cell carcinoma cells. PLoS One 2012;7:e48264. [Google Scholar] |
| 49. | Kroemer G. Autophagy: Adruggable process that is deregulated in aging and human disease. J Clin Invest 2015;125:1-4. [Google Scholar] |
Fulltext Views
4,666
PDF downloads
1,550





