Translate this page into:
Azathioprine-associated anagen effluvium
2 Department of Dermatology and STD, University College of Medical Sciences and Guru Teg Bahadur Hospital, New Delhi, India
Correspondence Address:
Sidharth Sonthalia
Skinnocence: The Skin Clinic and Research Centre, C-2246, Sushant Lok-1, Block-C, Gurgaon - 122 009, Haryana
India
| How to cite this article: Sonthalia S, Daulatabad D. Azathioprine-associated anagen effluvium. Indian J Dermatol Venereol Leprol 2016;82:322-324 |
Sir,
Anagen effluvium is the abrupt loss of anagen hair due to impaired mitotic activity of the hair follicle.[1] Chemotherapeutic agents such as paclitaxel, doxorubicin and cyclophosphamide constitute the common causes for anagen effluvium. We report an unusual case of anagen effluvium presenting within 48 h of initiation of therapy with azathioprine.
A 30-year-old patient with unstable vitiligo vulgaris presented to us with sudden loss of scalp hair in large bunches within 48 h of starting oral azathioprine 50 mg/day. She was already on oral betamethasone 3 mg twice a week and topical PUVAsol for the past 3 months. She reported regular menstrual cycles and denied a history of pruritus, pediculosis, boils, topical application over the scalp or chemical and cosmetic hair treatment. She used a mild shampoo twice a week. On examination, she had extensive non-cicatricial alopecia involving the middle two-thirds of the scalp extending from the frontal hair margin up to the vertex [Figure - 1]. There was no scalp erythema, flaking, crusts, nits, or lice. Eyebrows, eyelashes and body hair were intact. There were no clinical features of hyperandrogenism. The patient had brought a thick bunch of the lost hair for evaluation. [Figure - 2] Evaluation of this hair and hair extracted from hair pull test and trichogram revealed the predominance of tapered, irregular and pigmented hair suggestive of anagen hair with a few telogen hairs (57 anagen hair and 3 telogen hair (A:T ratio=19:1) of the 60 hair analysed). Trichoscopy revealed intact follicular openings and absence of yellow dots or exclamation mark hair. Hair shaft microscopy revealed a few dystrophic hair shafts [Figure - 3]. Hematological and biochemical investigations including hemogram, iron studies, serum levels of vitamin B12 and folate and thyroid function were normal except for borderline vitamin B12 deficiency. Anti-thyroid peroxidase and anti-parietal cell antibodies and antinuclear antibodies were not detectable. Venereal disease research laboratory (VDRL) test was non-reactive. The patient refused a scalp biopsy. Azathioprine was immediately stopped, the patient was counseled about the reversibility of her hair loss, suggested measures to minimize trauma to hair and was prescribed minoxidil 2% lotion for application twice a day along with a multivitamin supplement. She attained 80–90% regrowth within 6 months.
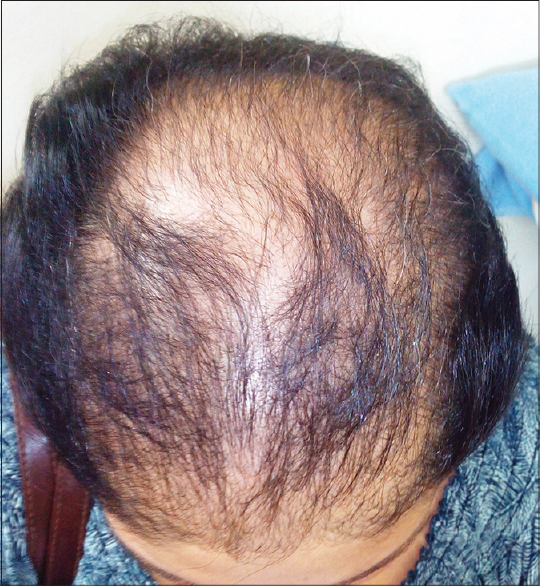 |
| Figure 1: Extensive non-cicatricial alopecia involving the middle twothird of the scalp extending from the frontal hair margin up to the vertex |
 |
| Figure 2: Bunch of hair extruded within 48 hours of intake of azathioprine |
 |
| Figure 3: Dystrophic anagen hair as detected on microscopy (×10 magnifi cation) |
The physiological shedding of hair that occurs due to the normal cycling of hair is primarily telogen hair and is limited to <100 hairs a day. Effluvium refers to active hair loss beyond this limit that persists for a period of 2–4 weeks.[1] Dysregulation of hair follicle cycling can result in both telogen effluvium and anagen effluvium. Anagen effluvium is the abrupt loss of anagen hair due to impaired mitotic activity of the hair follicle that usually begins 1–3 weeks after the insult.[1] Although chemotherapeutic agents constitute the most common cause, other factors including radiation, toxic chemicals and inflammatory diseases such as alopecia areata and pemphigus have been implicated.[1] Chemo-radiation impairs or disrupts the anagen cycle with resultant follicular dystrophy.[2] Since 80–90% of the scalp hair are in anagen, a large number of hair are abruptly lost in anagen effluvium. Fortunately, the quiescent bulge stem cells that re-initiate follicle growth are spared making the phenomenon completely reversible with regrowth usually apparent within 1–3 months.[3] Although anagen effluvium is mostly reversible, it is psychologically devastating for patients.
Azathioprine, an anti-metabolite that interferes with cellular DNA synthesis is used in post-solid organ transplant and as an immunosuppressive in various autoimmune disorders. We were able to find only 2 previous reports of azathioprine-induced anagen effluvium. In one case, the patient was receiving azathioprine for leukocytoclastic vasculitis whereas the other case had concomitant plica neuropathica with pancytopenia. Our case did not demonstrate any of these features.[4],[5] In both previous cases, the anagen effluvium developed within a month of starting azathioprine at the dose of 50 mg daily, whereas our patient reported to within 48 h. This is slightly atypical and may be explained by the fact that apart from the dose and duration of the anti-mitotic agent, anagen effluvium is also influenced by the synchronization of hair follicle cycling. It has been reported that in conditions with reduced duration of anagen such as androgenetic alopecia, even minor mitotic insult may lead to extensive hair loss.[1] However, our patient had not noticed any hair disorder prior to this. The exact role of the drug in the causation of such rapid hair loss could not be proved in our patient and could have been a mere association.
We believe that in view of the frequent use of azathioprine in different dermatoses, the treating dermatologists should be aware of this rare and distressing adverse effect of azathioprine.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Kanwar AJ, Narang T. Anagen effluvium. Indian J Dermatol Venereol Leprol 2013;79:604-12.
[Google Scholar]
|
| 2. |
Tosti A, Pazzaglia M. Drug reactions affecting hair: Diagnosis. Dermatol Clin 2007;25:223-31, vii.
[Google Scholar]
|
| 3. |
Trüeb RM. Diffuse hair loss. In: Blume-Peytavi U, Tosti A, Whiting DA, Trüeb RM, editors. Hair Growth and Disorders. Berlin: Springer; 2008. p. 259-72.
[Google Scholar]
|
| 4. |
Balasubramanian P, Jagadeesan S, Anjaneyan G, Thomas J. An interesting case report of azathioprine-induced anagen effluvium. Indian J Dermatol 2015;60:324.
[Google Scholar]
|
| 5. |
Joshi R, Singh S. Plica neuropathica (Plica polonica) following azathioprine-induced pancytopenia. Int J Trichology 2010;2:110-2.
[Google Scholar]
|
Fulltext Views
3,216
PDF downloads
1,237





