Translate this page into:
Bullous hemorrhagic dermatosis probably associated with enoxaparin
2 Department of Pathology, Hospital Ramon Y Cajal, E-28034 Madrid, Spain
Correspondence Address:
Laura Miguel-Gomez
Department of Dermatology, Hospital Ramon Y Cajal, Carretera de Colmenar Viejo, Km. 9, 100, 28034 Madrid
Sapin
| How to cite this article: Miguel-Gomez L, Fonda-Pascual P, Carrillo-Gijon R, Mu�oz-Zato E. Bullous hemorrhagic dermatosis probably associated with enoxaparin. Indian J Dermatol Venereol Leprol 2016;82:319-320 |
Sir,
Enoxaparin belongs to the category of low-molecular-weight heparins that are considered to have a lower incidence of secondary skin reactions. Bullous hemorrhagic dermatosis at sites distant from subcutaneous injections has been described in association with this therapy.
In July 2014, a 52-year-old man with a history of human immunodeficiency virus infection, hypertension, hypercholesterolemia and cirrhosis as a complication of hepatitis C was, in addition, diagnosed to have a multicentric hepatocellular carcinoma with brain metastases and venous thrombosis of the left sigmoid sinus and the left internal jugular vein. He was started immediately on enoxaparin, 100 IU/kg twice daily by subcutaneous injections. His other medications included enalapril, simvastatin and a combination of antiretroviral drugs (emtricitabine, tenofovir and efavirenz). Palliative chemotherapy was delayed until resolution of venous thrombosis. After a week of treatment with enoxaparin, the patient complained of non-pruritic hemorrhagic blisters on both arms and the left leg. He denied taking any other new medication and application of any topical product. Physical examination revealed five hemorrhagic tense blisters on both the arms and the left leg on otherwise normal skin [Figure - 1]. The mucosae were clinically normal. No lesions were detected at the enoxaparin injection sites. A coagulation profile and platelet count were normal. Skin biopsy revealed intraepidermal blisters containg red blood cells. No inflammatory infiltrate or signs of vasculitis were observed [Figure - 2]. Direct immunofluorescence did not showed any immunoreactants. A diagnosis of bullous hemorrhagic dermatosis, probably in association with enoxaparin subcutaneous injection, was made. Our differential diagnoses included porphyria cutanea tarda, bullous pemphigoid and acquired bullous epidermolysis; however, normal blood tests, direct immunofluorescence test and the histopathology ruled out these diagnoses. We excluded the others drugs (enalapril, simvastatin and antiretroviral drugs) because the patient had been taking them for many years, the only new drug was enoxaparin. The lesions resolved within days, despite continuation of treatment.
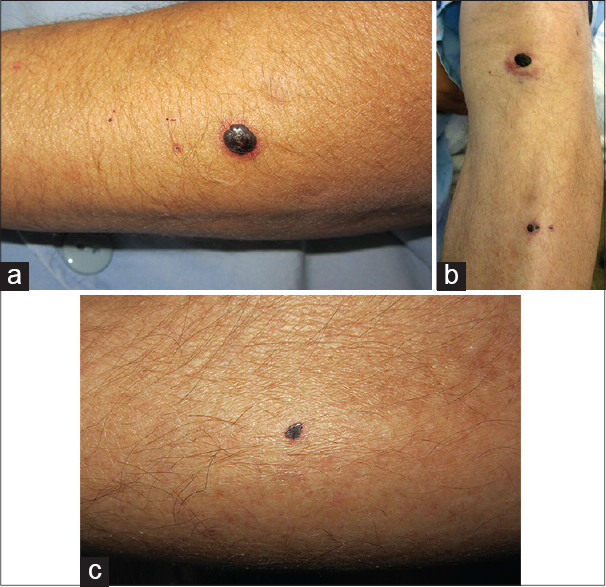 |
| Figure 1: (a) Hemorrhagic tense blister on the left arm on otherwise normal skin. (b) Hemorrhagic tense blister on the left leg. (c) Hemorrhagic tense blister on the right arm |
 |
| Figure 2: Intraepidermal blister containing red blood cells. No inflammatory infiltrate or signs of vasculitis are noted (H and E, original magnifi cation ×10) |
Enoxaparin is a class of anticoagulant that accelerates the activity of antithrombin III causing an inhibition of coagulation factors Xa and IIa. It is given as a subcutaneous injection. The drug is effective and safe for the prevention and treatment of venous thromboembolic disease and the treatment of unstable angina and acute myocardial infarction. Secondary skin reactions to this therapy are rare; the most frequent are skin necrosis, ecchymosis, urticaria, angioedema and contact dermatitis.[1],[2] The pathogenesis of these reactions is not well-understood.[3] Some authors have considered it a dose-dependent effect because it usually occurs when using unfractionated heparin or low-molecular-weight heparin at therapeutic doses.[1] Bullous hemorrhagic dermatosis usually appears some days after heparin injection although, rarely, it may occur weeks later. Clinically, it is characterised by tense hemorrhagic bullae distant from the heparin injection sites without any inflammatory signs in the adjacent skin. There are no other symptoms or signs. The key histopathological features of this entity are intraepidermal bullae with lack of thrombosis, vasculitis or inflammatory changes in vessels or dermis. These features are essential to exclude other cutaneous side effects of heparin injection.[1] Treatment for this condition is not necessary since the prognosis is usually favorable with spontaneous remission. Treatment with enoxaparin can be continued despite the eruption of hemorrhagic bullae. In conclusion, this case adds to the evidence from other reports that these drugs can probably cause skin reactions at sites distant from subcutaneous injections.[4],[5] Till date, there are less than 20 cases reported in the literature.[3] This disease is usually self-limited despite continuing the offending medication; hence, it may be an under-diagnosed phenomenon. Since enoxaparin is a drug frequently used in medical practice, dermatologists should be aware of these side effects so that their patients can be appropriately informed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Maldonado Cid P, Alonso de Celada RM, Noguera Morel L, Feito-Rodríguez M, Gómez-Fernández C, Herranz Pinto P. Cutaneous adverse events associated with heparin. Clin Exp Dermatol 2012;37:707-11.
[Google Scholar]
|
| 2. |
Schindewolf M, Lindhoff-Last E, Ludwig RJ, Boehncke WH. Heparin-induced skin lesions. Lancet 2012;380:1867-79.
[Google Scholar]
|
| 3. |
Maldonado Cid P, Moreno Alonso de Celada R, Herranz Pinto P, Noguera Morel L, Feltes Ochoa R, Beato Merino MJ, et al. Bullous hemorrhagic dermatosis at sites distant from subcutaneous injections of heparin: A report of 5 cases. J Am Acad Dermatol 2012;67:e220-2.
[Google Scholar]
|
| 4. |
Peña ZG, Suszko JW, Morrison LH. Hemorrhagic bullae in a 73-year-old man. Bullous hemorrhagic dermatosis related to enoxaparin use. JAMA Dermatol 2013;149:871-2.
[Google Scholar]
|
| 5. |
Concha-Garzon M, Sotomayor-Lopez E, Solano-Lopez G, Fraga J, De Argila D. Bullous hemorrhagic dermatosis distant from the site of heparin injection. Dermatol Online J 2014;20. pii: 13030/qt10w3j0ss.
[Google Scholar]
|
Fulltext Views
3,581
PDF downloads
1,390





