Translate this page into:
Assessment of intralesional injection of botulinum toxin type A injection for hypertrophic scars
Correspondence Address:
Alhasan M Elhefnawy
Department of Dermatology and Venereology, Faculty of Medicine, Ain Shams University, Cairo
Egypt
| How to cite this article: Elhefnawy AM. Assessment of intralesional injection of botulinum toxin type A injection for hypertrophic scars. Indian J Dermatol Venereol Leprol 2016;82:279-283 |
Abstract
Background: Hypertrophic scars are dermal fibroproliferative disorders that typically develop after a skin injury heals. They can cause physical, psychological, and cosmetic problems. The management of such scars remains a matter of debate due to lack of effective treatment methods and the inability to prevent recurrences. Recent reports have demonstrated that botulinum toxin type A improves wound healing so it may play a role in treating hypertrophic scars. Aims: We assessed the effectiveness of intralesional botulinum toxin type A injection for treating hypertrophic scars. Methods: This prospective clinical study included twenty patients with hypertrophic scars. Intralesional injection of botulinum toxin type A was given once a month for three months with a follow-up period of six months. Each lesion was injected until slight blanching occurred. Therapeutic satisfaction of the patient and physician were recorded. Lesions were assessed for erythema, itching and pliability. Each item was assessed on a 5-point scale. Results: Therapeutic satisfaction was recorded as 'good' in 14 patients and 'excellent' in the remaining six. The mean erythema score decreased from 3.2 to 1.0, the mean pliability score from 3.3 to 0.8 and the mean itching score from 2.7 to 0.7. All of these were statistically significant. Limitations: A larger sample size and longer follow-up period would have given a better evaluation but was not feasible due to the high expenses involved. Conclusion: Botulinum toxin type A is a novel and promising therapy for hypertrophic scars with few side effects.Introduction
Hypertrophic scars are dermal fibroproliferative disorders that usually develop after a skin injury heals.[1] Patients often experience physical deformities, restricted range of motion, pain and pruritus.[2] These scars are seen in about 50% of post surgical wounds and in more than 50% of healed deep burns.[3] Certain sites are predisposed because of increased skin tension, such as the chest and back.[4] These scars represent an aberration in the fundamental process of wound healing which includes hemostasis, inflammation, proliferation and the remodeling process.[5] Only hypertrophic scars have myofibroblasts with α smooth muscle actin expression which is believed to be an important element in the pathogenesis of contractures.[6] The exact etiopathogenesis of hypertrophic scars is still unknown.[7] The numerous treatments available include surgical excision, intralesional steroid injection, radiation therapy, lasers and pressure therapy, none of which offer satisfactory therapeutic results.
Botulinum toxin type A is an exotoxin of the anaerobic spore forming bacterium, Clostridium botulinum. It has become a useful tool for treating the hyperactive muscles of facial expression. It acts by inhibiting the exocytosis of acetylcholine which indirectly blocks neuromuscular transmission resulting in muscle flaccidity.[8] Although initial studies focussed on conditions related to skeletal muscle hyperactivity, it has become clear that botulinum toxin is effective in other disorders as well. It is being investigated for the treatment of smooth muscle conditions such as achalasia, anal fissure and overactive bladder. It is an accepted treatment for a few autonomic disorders such as primary hyperhidrosis. This led to the exploration of additional mechanisms of action, apart from the inhibition of acetylcholine release.[9] Several papers have reported that botulinum toxin type A can minimize facial scarring by reducing the muscle tension that acts on the healing wound. It may produce changes in the muscle spindles, that could lead to altered sensory input and changes in the cell cycle distribution of fibroblasts derived from the hypertrophic scar.[10],[11]
Methods
This study included twenty patients with hypertrophic scars who attended our dermatology outpatient clinic between May 2013 and November 2013. The study was assessed and approved by the institutional ethics committee. The procedure was explained to the patients and informed consent was obtained. All patients underwent history taking and clinical examination. Lesions that were present for more than one year and patients who were above the age of eighteen were included in the study. Exclusion criteria were pregnancy, lactation, local infection, patients on retinoids or anabolic steroids, severe systemic disease, pre-existing neuromuscular or cardiovascular disease, patients with unrealistic expectations or those who were treated for hypertrophic scars in the past.
Patients were treated with intralesional botulinum toxin type A as monotherapy. An intralesional injection of botulinum toxin type A (Botox Allergan ®, Irvine, CA, USA. 100 U vacuum-dried powder in a single-use vial for reconstitution diluted in 2 mL of sterile, preservative-free 0.9% saline to constitute a solution at a concentration of 4 U/0.1 mL) was administered once a month for a total period of three months. It was injected into the body of the scar with the help of a 9-gauge needle (0.3 mm × 0.8 mm) until slight blanching was visible. The dose was adjusted to 2.5 U/cm 3 of the lesion, not exceeding 100 units per se ssion. Photographs of the scars were taken before and immediately after each treatment session and at the 3rd and 6th month after the last treatment session.
At the 6th month follow-up period, the overall assessment information was obtained and graded subjectively on a 5-point scale as follows, 0: no improvement; 1: poor (up to 25% improvement); 2: fair (26–50% improvement); 3: good (51–75% improvement); or 4: excellent (76–100% improvement). The physician as well as the patient recorded this information. Erythema was graded on a 5-point scale as follows, 0: no erythema, 1: mild, 2: moderate, 3: severe, or 4: very severe. Lesion lightening was defined as the percentage of erythema reduction compared to the erythema before injection. Pliability (induration) also was graded on a 5-point scale as follows, 0: no induration, 1: mild, 2: moderate, 3: severe, or 4: very severe. Lesion softening was defined as the percentage of pliability reduction compared to the pliability before injection. The severity of itching was graded on a 5-point scale as follows, 0: no itching, 1: mild, 2: moderate, 3: severe, or 4: very severe. Improvement in itching was defined as the percentage of itching reduction compared to the itching before injection.
During the six month follow-up period, the patients were monitored for the development of any complications such as pain, bleeding, edema, erythema, hypopigmentation, hyperpigmentation, secondary infection and recurrence.
Statistical analysis
The data was analyzed using Statistical Package for Social Science version 20 (SPSS Inc., Chicago, IL, USA). Parametric data were expressed as mean ± standard deviation and nonparametric data were expressed as numbers and percentages of the total. Comparisons of the pre-treatment and post-treatment mean ± standard deviation were done using the paired t-test. A P value of P < 0.05 was considered statistically significant.
Results
The study enrolled 14 men and 6 women with a mean age of 23.9 ± 5.4 years (range 19–35 years). The mean duration of the hypertrophic scars was 1.9 ± 0.84 years (range 1–3 years), and the mean lesion size was 2.85 ± 1.4 cm 3 (range 1.25–6.0 cm 3). There were six cases of hypertrophic scars on the face and neck, four on the chest and shoulders and the remaining ten were on the extremities [Table - 1].

According to the overall assessment of clinical response by the physician, 14 lesions had “good” improvement, and 6 lesions had “excellent” improvement. Patient assessment showed “good” improvement in 12 lesions and “excellent” improvement in 8 lesions. There was a significant difference in the mean erythema scores from the pre-treatment (3.2 ± 0.78) to post-treatment stage (1.0 ± 0.66) (P = 0.000) [Table - 2], [Figure - 1] and [Figure - 2].

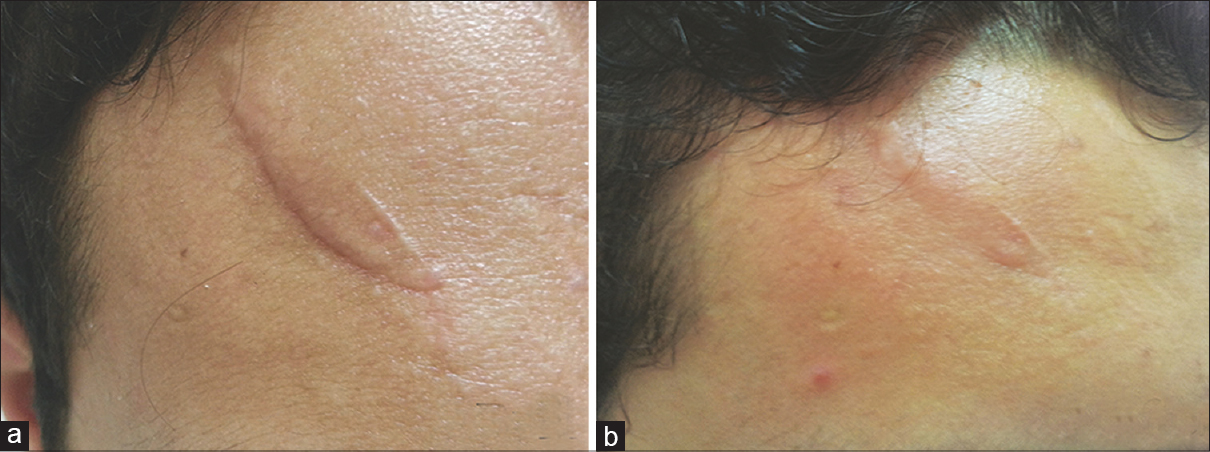 |
| Figure 1: (a) Scar on the forehead before giving botulinum toxin type A intralesional injection. (b) Scar after 6 months of treatment |
 |
| Figure 2: (a) Scar on the chest before giving botulinum toxin type A intralesional injection. (b) Scar after 6 months of treatment |
There was no recurrence or complications in any of the lesions during the 6-month follow-up period.
Discussion
Many researchers have tried to treat hypertrophic scars by reducing excess collagen with the least amount of complications or recurrence rates. Our study demonstrates the ability of botulinum toxin type A injection to significantly decrease erythema, induration and itching with an improvement in the overall appearance.
Botulinum toxin type A acts by blocking cholinergic neuromuscular transmission or cholinergic autonomic innervation of exocrine glands and smooth muscles. It inhibits the action of soluble N-ethylmaleimide-sensitive factor attachment protein receptor protein, which is involved in the fusion of acetylcholine-containing synaptic vesicles with the plasma membrane.[12] The action of botulinum toxin type A on hypertrophic scars is believed to be a temporary denervation of smooth muscle fibers decreasing the tension in the scar tissue. This is one of the main factors that determine the degree of scar formation.[13] Hypertrophic scars result from fibroblast proliferation which is responsible for the enhanced collagen production that leads to excessive scarring.[14] By decreasing the tension in the scar, botulinum toxin causes local fibroblasts to gradually change their functional status to proliferate slower, secrete less biologically active mediators and synthesize less extracellular matrix and collagen resulting in improvement of hypertrophic scars.[15]
Recent studies have elucidated the molecular connections between the biological mechanisms of botulinum toxin type A and hypertrophic scars. An in vitro study by Zhibo and Miaobo showed that a higher percentage of fibroblasts were in the non-proliferative phases (G0 and G1) when treated with botulinum toxin type A (64%) as compared to the control group (36%).[11] They also confirmed the ability of botulinum toxin type A to reduce the expression of transforming growth factor β 1 in keloid fibroblasts.[16] Transforming growth factor β 1 is considered to be the main regulator in the pathogenesis of hypertrophic scars and is associated with excessive deposition of scar tissue and fibrosis.[17] Another in vitro study demonstrated that botulinum toxin type A could promote apoptosis, inhibit transforming growth factor β 1 protein expression, affect the cell cycle and cell dynamics to inhibit the proliferation of fibroblasts derived from hypertrophic scars.[11] Botulinum toxin type A also inhibits the release of substance P which requires the soluble N-ethylmaleimide-sensitive factor attachment protein receptor protein activity. This contributes to the improvement of erythema, pain, and itching.[18],[19] However, another study showed that the expression of cytokines and growth factors in human keloid tissue was unaffected by botulinum toxin type A incubation suggesting that there was no significant therapeutic role for botulinum toxin type A injections in keloid treatment.[20]
Clinically, several studies have confirmed the positive effect of intralesional botulinum toxin type A in hypertrophic scars.[21] Xiao et al. treated 19 patients with hypertrophic scars once monthly with intralesional botulinum toxin type A for 3 months, with a follow-up for at least 6 months.[22] Twelve patients noted that the improvement of their lesions was “good,” and the other seven said that the improvement was “excellent.” According to the overall assessment by the plastic surgeons, there was “good” improvement in fifteen lesions and “excellent” improvement in the remaining four. The mean erythema score decreased from 3.41 to 1.23, the pliability score decreased from 3.85 to 0.78, and the itching score decreased from 3.50 to 0.83. Zhibo and Miaobo have studied intralesional injection with botulinum toxin type A at a concentration of 35 U/mL (range 70–140 U/session) with 3-month intervals for a maximum of 9 months in twelve patients.[16] At 1-year of follow-up, three patients demonstrated excellent improvement, five had good improvement and the remaining four had fair improvement. There were no signs of recurrence during the follow-up period. In contrast, Gauglitz et al. determined that repeated injections with botulinum toxin type A for up to six months did not change the macroscopic appearance, morphology or size of scars in four patients. They had used optical profilometry to confirm the visual impression.[23]
Limitations
A larger sample size and longer follow-up period would have given a better evaluation, but was not feasible due to the high cost of the study.
Conclusion
This study concludes that monthly injections of intralesional botulinum toxin type A for a period of three months is associated with an improvement of erythema, pliability, and itching in hypertrophic scars. It is a novel and promising therapeutic agent with no side effects noted so far.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Meenakshi J, Jayaraman V, Ramakrishnan KM, Babu M. Keloids and hypertrophic scars: A review of literature. Indian J Plast Surg 2005;38:175-9.
[Google Scholar]
|
| 2. |
Gassner HG, Sherris DA, Otley CC. Treatment of facial wounds with botulinum toxin A improves cosmetic outcome in primates. Plast Reconstr Surg 2000;105:1948-53.
[Google Scholar]
|
| 3. |
Rodero MP, Khosrotehrani K. Skin wound healing modulation by macrophages. Int J Clin Exp Pathol 2010;3:643-53.
[Google Scholar]
|
| 4. |
Yagi KI, Dafalla AA, Osman AA. Does an immune reaction to sebum in wounds cause keloid scars? Beneficial effect of desensitisation. Br J Plast Surg 1979;32:223-5.
[Google Scholar]
|
| 5. |
Rahban SR, Garner WL. Fibroproliferative scars. Clin Plast Surg 2003;30:77-89.
[Google Scholar]
|
| 6. |
Baur PS, Larson DL, Stacey TR. The observation of myofibroblasts in hypertrophic scars. Surg Gynecol Obstet 1975;141:22-6.
[Google Scholar]
|
| 7. |
Shaffer JJ, Taylor SC, Cook-Bolden F. Keloidal scars: A review with a critical look at therapeutic options. J Am Acad Dermatol 2002;46 2 Suppl: S63-97.
[Google Scholar]
|
| 8. |
Naumann M, So Y, Argoff CE, Childers MK, Dykstra DD, Gronseth GS, et al. Assessment: Botulinum neurotoxin in the treatment of autonomic disorders and pain (an evidence-based review): Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2008;70:1707-14.
[Google Scholar]
|
| 9. |
Aoki K. Pharmacology, immunology and current developments. In: Benedetto A, editor. Botulinum Toxin in Clinical Dermatology. Vol. 3 Ch. 1. UK: Taylor and Francis; 2006. p. 16.
[Google Scholar]
|
| 10. |
Aoki KR. Botulinum toxin: A successful therapeutic protein. Curr Med Chem 2004;11:3085-92.
[Google Scholar]
|
| 11. |
Zhibo X, Miaobo Z. Botulinum toxin type A affects cell cycle distribution of fibroblasts derived from hypertrophic scar. J Plast Reconstr Aesthet Surg 2008;61:1128-9.
[Google Scholar]
|
| 12. |
Hackett R, Kam PC. Botulinum toxin: Pharmacology and clinical developments: A literature review. Med Chem 2007;3:333-45.
[Google Scholar]
|
| 13. |
Scheffer AR, Erasmus C, van Hulst K, van Limbeek J, Jongerius PH, van den Hoogen FJ. Efficacy and duration of botulinum toxin treatment for drooling in 131 children. Arch Otolaryngol Head Neck Surg 2010;136:873-7.
[Google Scholar]
|
| 14. |
Nordlund JJ. Keloids and hypertrophic scars. Arch Dermatol 2000;137:77.
[Google Scholar]
|
| 15. |
Xiao Z, Qu G. Effects of botulinum toxin type a on collagen deposition in hypertrophic scars. Molecules 2012;17:2169-77.
[Google Scholar]
|
| 16. |
Zhibo X, Miaobo Z. Intralesional botulinum toxin type A injection as a new treatment measure for keloids. Plast Reconstr Surg 2009;124:275e-7e.
[Google Scholar]
|
| 17. |
Xiao Z, Zhang F, Lin W, Zhang M, Liu Y. Effect of botulinum toxin type A on transforming growth factor beta1 in fibroblasts derived from hypertrophic scar: A preliminary report. Aesthetic Plast Surg 2010;34:424-7.
[Google Scholar]
|
| 18. |
Gassner HG, Brissett AE, Otley CC, Boahene DK, Boggust AJ, Weaver AL, et al. Botulinum toxin to improve facial wound healing: A prospective, blinded, placebo-controlled study. Mayo Clin Proc 2006;81:1023-8.
[Google Scholar]
|
| 19. |
Maderal AD, Tang JC, Vivas AC, Viera MH. Hypertrophic scars and keloids, part 2: Newer and investigational therapies. Cosmet Dermatol 2012;25:373-9.
[Google Scholar]
|
| 20. |
Haubner F, Leyh M, Ohmann E, Sadick H, Gassner HG. Effects of botulinum toxin A on patient-specific keloid fibroblasts in vitro. Laryngoscope 2014;124:1344-51.
[Google Scholar]
|
| 21. |
Viera MH, Amini S, Valins W, Berman B. Innovative therapies in the treatment of keloids and hypertrophic scars. J Clin Aesthet Dermatol 2010;3:20-6.
[Google Scholar]
|
| 22. |
Xiao Z, Zhang F, Cui Z. Treatment of hypertrophic scars with intralesional botulinum toxin type A injections: A preliminary report. Aesthetic Plast Surg 2009;33:409-12.
[Google Scholar]
|
| 23. |
Gauglitz GG, Bureik D, Dombrowski Y, Pavicic T, Ruzicka T, Schauber J. Botulinum toxin A for the treatment of keloids. Skin Pharmacol Physiol 2012;25:313-8.
[Google Scholar]
|
Fulltext Views
6,868
PDF downloads
1,695





