Translate this page into:
A randomized placebo-controlled double-blind pilot study of methotrexate in the treatment of H1 antihistamine-resistant chronic spontaneous urticaria
2 Centre for Community Medicine, All India Institute of Medical Sciences (AIIMS), New Delhi, India
Correspondence Address:
Vinod K Sharma
Departments of Dermatology, Venereology, and Leprology, All India Institute of Medical Sciences (AIIMS), New Delhi - 110 029
India
| How to cite this article: Sharma VK, Singh S, Ramam M, Kumawat M, Kumar R. A randomized placebo-controlled double-blind pilot study of methotrexate in the treatment of H1 antihistamine-resistant chronic spontaneous urticaria. Indian J Dermatol Venereol Leprol 2014;80:122-128 |
Abstract
Background: Chronic urticaria not responsive to antihistamines is a difficult disease to manage. Methotrexate has been used in difficult chronic urticarias with some benefit. Objective: To evaluate the efficacy of methotrexate in the treatment of chronic spontaneous urticaria poorly responsive to H1 antihistaminics. Methods: In a randomized double-blind trial at the Department of Dermatology and Venereology of a tertiary care centre, 29 patients with chronic spontaneous urticaria not responding well to H1 antihistaminics were recruited. Patients were randomly allocated to receive either a weekly dose of oral methotrexate 15 mg or placebo (calcium carbonate) for a total duration of 12 weeks, after which treatment was stopped and patients were followed up for relapse of urticaria. Each group also received levocetrizine 5 mg once daily for symptom control. Primary outcome measured was a reduction by >2/3 rd of baseline urticaria scores after 12 week therapy. Secondary outcome was a reduction in antihistamine requirement after stopping therapy. Results: Fourteen patients were randomized to the methotrexate group and fifteen patients to the placebo group. Out of 17 patients who completed therapy, the primary outcome was achieved by 3.5 ± 1.9 (out of 10) patients in the methotrexate group and by 3.67 ± 1.03 (out of 7) patients in the placebo group (P > 0.05). Ten patients followed up, after stopping therapy, for a mean period of 3.5 ± 2.4 months; 3 remained in remission and 7 had relapsed. One patient had uncontrollable nausea and vomiting after taking methotrexate and was withdrawn from the study. The placebo group did not experience any side effects. Conclusions: Methotrexate 15 mg weekly for 3 months did not provide any additional benefit over H1 antihistamines in this study but an adequately powered study with longer follow up is required to assess its utility.Introduction
Urticaria is a common dermatological condition affecting an estimated 15-20% of the general population at least once during the life time. [1] Chronic urticaria is defined by recurrent episodes occurring at least twice a week for 6 weeks or more. [2]
Diagnosis of chronic urticaria is primarily clinical, hence it requires only guided investigations based on detailed clinical history and examination. [3] Second generation non-sedating antihistaminics (like levocetrizine, desloratidine, fexofenadine etc.) are the mainstay of therapy and are preferred over the sedating ones due to their good safety and tolerability profile. Often in clinical practice antihistaminics can be used in upto 2-4 fold higher doses than recommended for managing difficult or poorly responsive urticaria. [4],[5],[6] Other therapeutic strategies for difficult or resistant urticaria include adding leukotriene antagonists, corticosteroids, cyclosporine, methotrexate, mycophenolate mofetil, cyclophosphamide, intravenous immunoglobulin, omalizumab, tacrolimus and narrow band UVB. [7],[8],[9],[10],[11],[12],[13],[14],[15],[16] Methotrexate is frequently used in dermatology and has been claimed to be effective in chronic urticarias of the idiopathic, autoimmune and steroid dependant types. [10],[17],[18],[19] We conducted a double blind placebo controlled randomized trial with the hypothesis that addition of methotrexate would reduce symptoms of H1 antihistamine resistant urticaria as well as avoid relapse of H1 antihistamine resistant urticaria after stopping antihistamine therapy.
Methods
Study design
It was a randomized double blind placebo controlled trial conducted on consecutive patients of chronic urticaria presenting to the dermatology outpatient department of the All India Institute of Medical Sciences (AIIMS) New Delhi. This study was approved by our institute′s ethics committee. One dermatologist (MK) administered the therapy to the patients and the follow up evaluation was done by another one (VKS).
Patients
Almost all previous studies showed good response to addition of methotrexate to resistant/steroid dependant urticaria. Hence, keeping the α error as 0.05, power of the study 0.80 and taking the treatment efficacy of methotrexate at approximately. 80% based on available literature [18],[19] and assuming a clearance rate of 33% in the control (antihistamine alone) group, a sample size of 14 patients was required in each group. An allocation sequence was generated (by MR) using a table of random numbers with an allocation ratio of 1:1. Allocation was concealed by using consecutively numbered opaque envelopes. Informed written consent was taken from all study participants. Antihistamine-resistant chronic spontaneous urticaria was defined as those on 5 mg of levocetrizine or 10 mg of cetirizine twice a day for 15 days and a combination of fexofenadine 180 mg and hydroxyzine 25 mg for another 15 days without >50% reduction of baseline urticaria activity scores. Antihistamines were subsequently stopped for 1 week to establish baseline urticaria scores in all cases prior to inclusion in the study. Patients were excluded if they had urticaria for less than 6 weeks or had urticaria solely due to foods, drugs, physical and environmental factors, infections and infestations, and urticarial vasculitis. They were also excluded in the following situations: Pregnant/lactating women, women wanting to conceive or men wanting to father within 6 months of entry, patients having any systemic disease, alcoholism and patients who had taken any immunosuppressive agent within 4 weeks of entry.
Treatment
Patients were recruited from July 2008 to December 2010. At each monthly visit, they were given numbered envelopes containing hard shelled capsules with methotrexate or placebo. Case group (group I) was treated with capsules containing powdered methotrexate 15 mg per week for 3 months. Control group (group II) was treated with identical capsules containing placebo (powdered calcium carbonate) for 3 months. Each group was allowed to take levocetrizine 5 mg daily or as required for symptom control. No other antihistaminies were allowed.
Pre-treatment clinical evaluation
A thorough history was taken and a complete clinical examination was done. Urticaria severity assessment was done using mean number of wheals/day, [20] mean pruritus score, [20] average size of wheal (0 to 3, for sizes 0 to >3inches), duration of the wheal (0 to 4, for durations <4 hours to >24 hours), wheal episodes per day >1 hour apart (0 to 3, for 0 to >3 episodes) and days with urticaria per week.
Pre-treatment laboratory evaluation
Hemogram, liver and renal function tests, urine and stool examination for ova/cyst and occult blood were carried out. Autologous serum skin test (ASST) was also carried out at baseline by the standard method. [21]
Assessment of response to treatment
Patients were clinically evaluated at 2, 4, 8 and 12 weeks to determine the improvement in severity of urticaria and itching. Blood parameters (hemogram, liver and kidney function tests) were repeated at 2, 6 and 12 weeks. Any clinical and laboratory abnormalities were noted.
In the post-treatment follow-up, complete remission was defined as absence of urticarial wheals and pruritus off antihistamines. Partial remission was defined as reduction in wheals and pruritus as compared to baseline but with persisting symptoms requiring antihistaminies for relief. Relapse was defined as recurrence of wheals after treatment stoppage with a need to continue antihistamine therapy for symptomatic relief..
Primary outcome measured was a reduction by >2/3 rd of baseline urticaria scores (i.e., a reduction of more than 66.7%) during therapy.
Secondary outcome was a reduction in antihistamine requirement after stopping therapy.
Statistical analysis
Data was analyzed using SPSS 19.0 (SPSS Inc., Chicago, USA). It was reported as mean or proportion wherever appropriate. Mann-Whitney U test was used to test the significance of difference in mean between two groups, whereas Wilcoxon Rank Sum test was used to assess the significance of difference in mean scores at 0 weeks and 12 weeks within a group. Chi-Square test was used to test the significance of difference in the proportions. The level of significance was assumed as 5%. Both intention to treat and per protocol analysis were done for all the variables.
Results
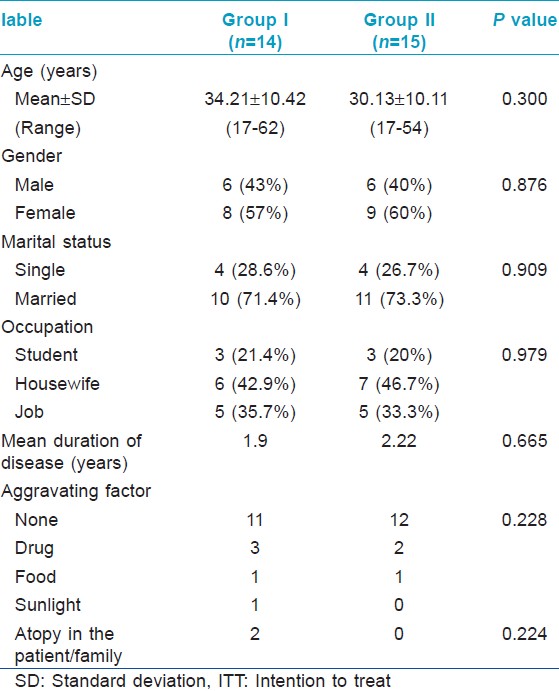
[Figure - 1] illustrates the recruitment, allocation and analysis of patients. Baseline demographic and clinical characteristics of study subjects in the two groups were comparable [Table - 1]. A total of 29 patients were recruited. Twelve patients did not complete the 12 week treatment course; 8 patients in the placebo group and 4 patients in the methotrexate group. Out of these, 5 patients (4 in placebo group, 1 in methotrexate group) did not start treatment after enrolling in the study and 6 patients (4 in placebo group, 2 in methotrexate group) were not satisfied with the treatment. One patient in the methotrexate group was withdrawn from treatment due to side effects.
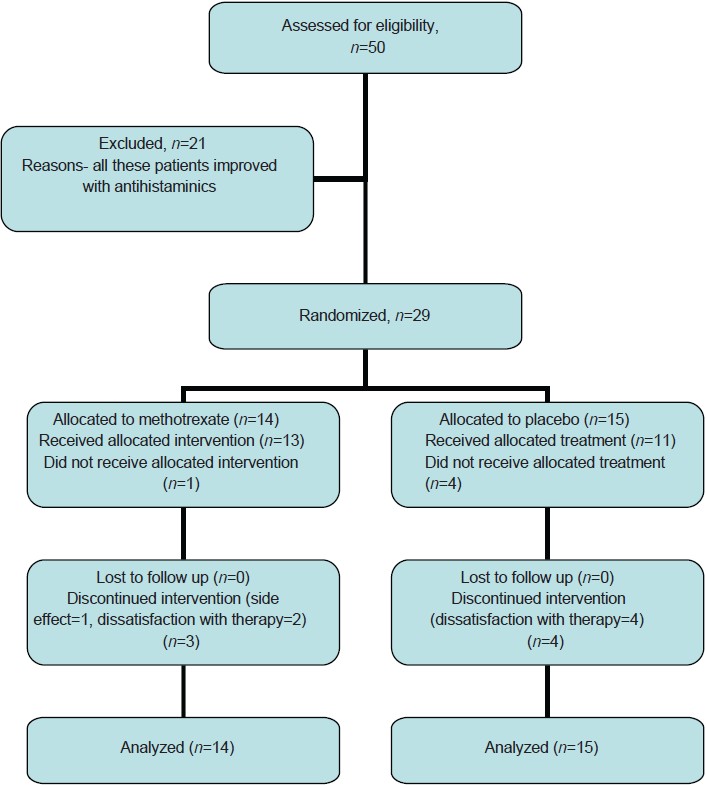 |
| Figure 1: Flow diagram of patients in the study |
Evaluation of efficacy of the intervention in each group was done using the clinical parameters described above. [Table - 2] shows the intention to treat analysis. Overall, all clinical parameters showed statistically significant improvement in both groups on intra-group analysis. Per protocol analysis [Table - 3] showed that other than wheal size in the placebo group, all other parameters in both groups improved significantly at the end of 12 weeks as compared to baseline. But when the two groups were compared with each other, there was no statistical difference. Mean wheal score in the two groups, as calculated at each visit, has been depicted in [Figure - 2]. Overall, the primary outcome in the form of >2/3 rd (>66.7%) reduction in the scores at the end of 12 weeks was achieved by 3.5 ± 1.9 (out of 10) patients in the methotrexate group and by 3.67 ± 1.03 (out of 7) patients in the placebo group. In the context of primary outcome in reduction of the various scores, methotrexate was better than placebo only in reducing the days with urticaria per week. In all other parameters, methotrexate was less effective, although the difference was not statistically significant.
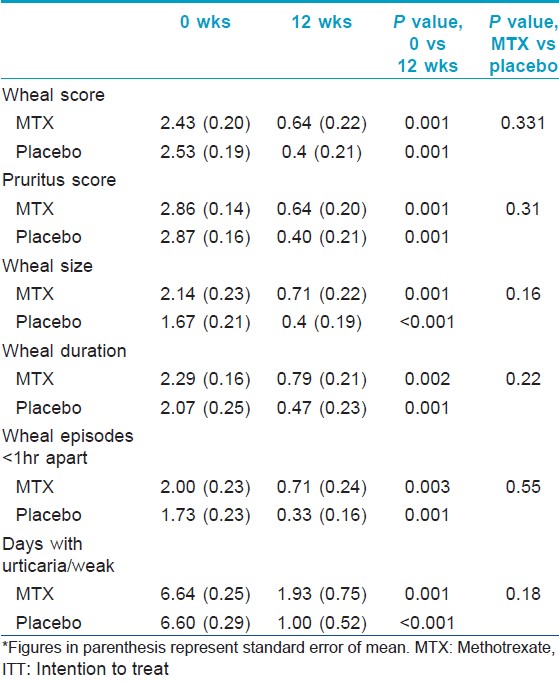
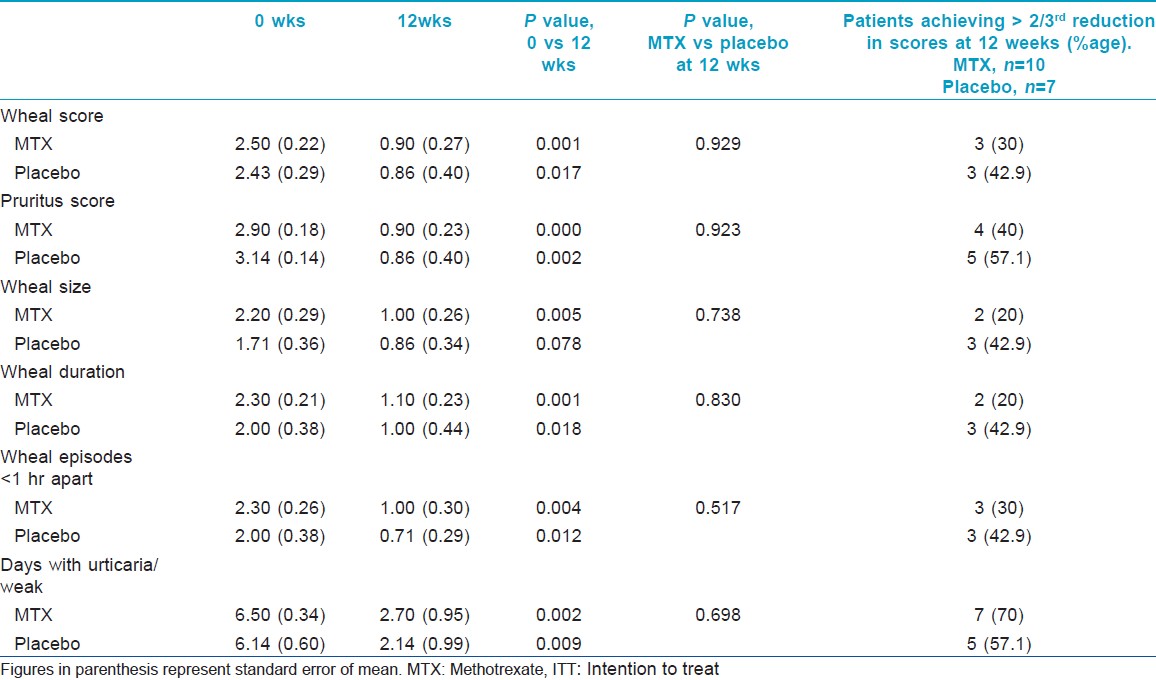
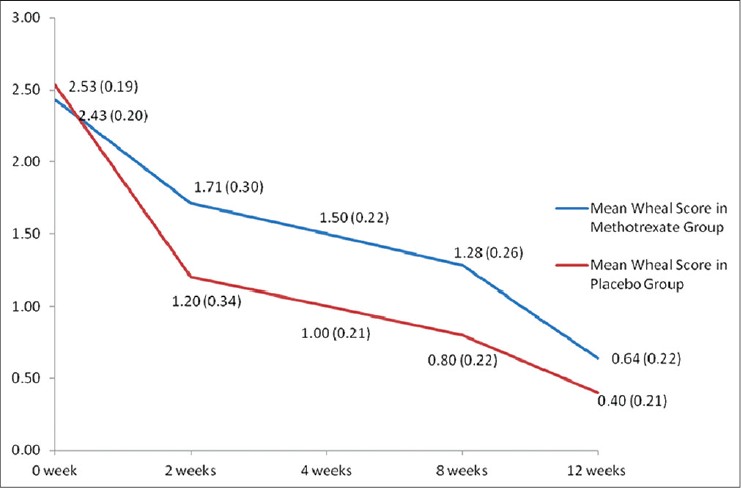 |
| Figure 2: Comparison of mean wheal scores between the two groups (group 1 = methotrexate + levocetrizine 5 mg daily; and group 2 = placebo + levocetrizine 5 mg daily). *Figures in parenthesis represent standard error of mean |
Autologous serum skin test was positive in 14 (48.3%) patients, 6 in placebo and 8 in methotrexate group. Out of these, 3 patients in placebo and 7 patients in methotrexate group completed 12 weeks treatment. A significant (>66.7%) reduction in mean wheal scores and mean pruritus scores in the methotrexate group was achieved in 30% and 40% of the ASST-negative patients and in 14.2% and 28.4% of the ASST-positive patients, respectively. On the other hand, ASST-positive patients showed better reduction in these scores as compared to the ASST-negative patients in the placebo group (66.7% each versus 57.1% and 42.9%, respectively).
Ten patients followed up after discontinuation of therapy. The mean follow up period was 3.5 ± 2.4 months (0.5-9 months). Three patients had remission; 2 had partial remission (reduction in antihistamine requirement) one patient each belonging to placebo and methotrexate group and one patient in the methotrexate group had complete remission (off antihistamines, no recurrence of lesions/symptoms) at 6 months. Rest of the patients relapsed immediately after stoppage of therapy; full or hiked up dosage of H1 antihistaminics were required to control the symptoms.
No life-threatening therapy related adverse effects were noted. The placebo group did not report any side effects. One patient in the methotrexate group was withdrawn from the study due to severe nausea/vomiting uncontrolled with antiemetics (domperidone 20 mg) and improved after the drug was discontinued. She did not experience any laboratory derangements. Two patients in the methotrexate group showed a rise in transaminase levels; one showed a mild (1.5x) rise at 8 weeks which improved spontaneously despite continuation of therapy and did not recur, and another showed 3x rise at 12 weeks, i.e., at the end of therapy but other investigations were within normal limits (bilirubin, viral markers, ultrasound abdomen) and the levels returned to normal 7 days later.
Discussion
Management of chronic urticaria is time consuming and frustrating and has high direct and indirect healthcare costs with socio-economic implications due to reduction in performance by almost 20-30%. [20],[22] Resistant urticaria has been variously defined in literature as a steroid dependant rash poorly responsive to Multiple antihistamies and immunosuppressive agents. [18] Okubo et al., defined patients not responding to 10 mg cetirizine per day for 1 week as having resistant urticaria. [23] European Union consensus guidelines define resistant urticaria as one which is not controlled after updosing of non-sedating H1 antihistaminics to 4 times the usual dose, but they indicate that this recommendation is based on low quality evidence. [24] Hence, in the absence of a standard definition, we used our own definition of resistant urticaria. Several immunosuppressive and immunomodulatotory agents have been tried in the treatment of resistant urticarias, out of which cyclosporine is the most well studied. In a randomized controlled trial on 99 patients with chronic idiopathic urticaria, among three treatment arms treated for 16 weeks each (cyclosporine, cyclosporine with placebo and placebo), there was significant improvement in symptom scores in cyclosporine groups compared with placebo, starting at 8 weeks of therapy and this improvement was maintained even 8 weeks after stopping therapy. [9]
Similarly, a RCT evaluated single dose omalizumab in 90 patients of H1 antihistamine refractory chronic idiopathic urticaria. Only the 300 mg omalizumab group and the 600 mg omalizumab group showed greater improvement versus the placebo group in urticaria activity scores. Onset of effect with omalizumab occurred after 1 to 2 weeks. Omalizumab was well tolerated, and the incidence of adverse events was similar across treatment groups. [14] Similarly in a recent phase 3 RCT, omalizumab 150 mg and 300 mg once a month was found to improve chronic urticaria significantly better than placebo and was well tolerated. [25]
A few reports on the usefulness of methotrexate in therapy resistant chronic urticaria have been previously published. Perez et al., [18] carried out a retrospective review of 16 patients of steroid dependant chronic urticaria (10 chronic urticaria, 4 urticarial vasculitis and 2 angioedema) treated with methotrexate in doses ranging from 5-15 mg/week. Twelve out of 16 patients responded to therapy, seven being able to taper their oral steroids and 2 were able to discontinue steroids completely. Onset of therapeutic effect of methotrexate was noted at periods varying from 3 weeks to more than 6 months. The only reported side effects included hair thinning and fatigue. Follow up was not reported by the authors. Sagi et al.,[19] reported that out of 8 patients with chronic urticaria who had responded poorly to antihistaminies, oral steroids and other immunosuppressants and subsequently treated with weekly 15-25 mg methotrexate, 7 showed complete remission with onset of effect seen at 3-5 weeks, maintained for 2-15 months. None of these reports have mentioned whether these patients could be weaned off antihistaminies as well.
Hiking the dose of H1 antihistaminies may produce significant improvement in chronic urticaria. [4],[5],[6] However, larger well-powered studies are required to conclusively prove this claim. In keeping with the equivocal results of our RCT, it may be worthwhile to further investigate methotrexate and other immunosuppressants and compare them with hiked up dosing of H1 antihistamines for uncontrolled chronic spontaneous urticaria.
We found that 15 mg weekly methotrexate for 12 weeks perhaps does not provide any additional benefit over and above anti-H1 antihistaminics in the treatment of resistant chronic spontaneous urticaria. Also, antihistamines were safe as compared to methotrexate in terms of drug related side effects. As far as ascertained, an RCT evaluating methorexate in the treatment of chronic urticaria has not been Reported in English literature till date.
Previous open label studies have mentioned that although ASST-positive patients benefit from immunosuppressive therapy, a successful outcome is not dependant on demonstration of auto-reactivity. [21],[26] Similarly from our study, we could not specifically comment whether or not ASST status affecting response to treatment since placebo group patients had better reduction in scores than methotrexate group irrespective of their ASST status.
Short duration of treatment was one of the limitations of our study. This might explain why a large proportion of the patients relapsed after stoppage of therapy. Another limitation was the large drop out (12 out of 29 patients) which could not be replaced leaving the study underpowered. Since existing literature suggests its beneficial effect in other forms of chronic urticaria, larger placebo controlled randomized trials should be conducted to study the role of in the therapy of chronic spontaneous urticaria.
Acknowledgments
We thank Dr. Uttam Kumar (ex-senior Resident) and Dr. Smita Nagpal (ex-senior research fellow), department of dermatology, AIIMS, for helping with the design and recruitment of patients in the study.
| 1. |
Caliskaner Z, Ozturk S, Turan M, Karaayvaz M. Skin test positivity to aeroallergens in the patients with chronic urticaria without allergic respiratory disease. J Investig Allergol Clin Immunol 2004;14:50-4.
[Google Scholar]
|
| 2. |
Grattan CEH, Sabroe RA, Greaves MW. Chronic urticaria. J Am Acad Dermatol 2002;46:645-57.
[Google Scholar]
|
| 3. |
Kozel MM, Mekkes JR, Bossuyt PM, Bos JD. The effectiveness of a history-based diagnostic approach in chronic urticaria and angioedema. Arch Dermatol 1998;134:1575-80.
[Google Scholar]
|
| 4. |
Siebenhaar F, Degener F, Zuberbier T, Martus P, Maurer M. High-dose desloratadine decreases wheal volume and improves cold provocation thresholds as compared with standard dose treatment in patients with acquired cold urticaria: A randomized, placebo-controlled, crossover study. J Allergy Clin Immunol 2009;123:672-9.
[Google Scholar]
|
| 5. |
Godse KV. Updosing of antihistamines to improve control of chronic urticaria. Indian J Dermatol Venereol Leprol 2010;76:61-2.
[Google Scholar]
|
| 6. |
Kameyoshi Y, Tanaka T, Mihara S, Takahagi S, Niimi N, Hide M. Increasing the dose of cetirizine may lead to better control of chronic idiopathic urticaria: An open study of 21 patients. Br J Dermatol 2007;157:803-4.
[Google Scholar]
|
| 7. |
Di Lorenzo G, Pacor ML, Mansueto P, Esposito Pellitteri M, Lo Bianco C, Ditta V, et al. Randomized placebo controlled trial comparing desloratadine and montelukast in monotherapy and desloratadine plus montelukast in combined therapy for chronic idiopathic urticaria. J Allergy Clin Immunol 2004;114:619-25.
[Google Scholar]
|
| 8. |
Asero R, Tedeschi A. Usefulness of a short course of oral prednisone in antihistamine-resistant chronic urticaria: A retrospective analysis. J Investig Allergol Clin Immunol 2010;20:386-90.
[Google Scholar]
|
| 9. |
Vena GA, Cassano N, Colombo D, Peruzzi E, Pigatto P; Neo-I-30 Study Group. Cyclosporine in chronic idiopathic urticaria: A double blind, randomized, placebo-controlled trial. J Am Acad Dermatol 2006;55:705-9.
[Google Scholar]
|
| 10. |
Gach JE, Sabroe RA, Greaves MW, Black AK. Methotrexate-responsive chronic idiopathic urticaria: A report of 2 cases. Br J Dermatol 2001;145:340-3.
[Google Scholar]
|
| 11. |
Zimmerman AB, Berger EM, Elmariah SB, Soter NA. The use of mycophenolate mofetil for the treatment of autoimmune and chronic idiopathicurticaria: Experience in 19 patients. J Am Acad Dermatol 2012;66:767-70.
[Google Scholar]
|
| 12. |
Asero R. Oral cyclophosphamide in a case of cyclosporin and steroid-resistant chronic urticaria showing autoreactivity on autologous serum skin testing. Clin Exp Dermatol 2005;30:578-602.
[Google Scholar]
|
| 13. |
Mitzel-Kaoukhov H, Staubach P, Müller-Brenne T. Effect of high-dose intravenous immunoglobulin treatment in therapy-resistant chronic spontaneous urticaria. Ann Allergy Asthma Immunol 2010;104:253-8.
[Google Scholar]
|
| 14. |
Saini S, Rosen KE, Hsieh HJ, Wong DA, Conner E, Kaplan A et al. A randomized, placebo controlled, dose ranging study of single dose omalizumab in patients with H1 antihistamine refractory chronic idiopathic urticaria. J Allergy Clin Immunol 2011;128:567-73.e1.
[Google Scholar]
|
| 15. |
Kessel A, Bamberger E, Toubi E. Tacrolimus in the treatment of severe chronic idiopathic urticaria: An open label prospective study. J Am Acad Dermatol 2005;52:145-8.
[Google Scholar]
|
| 16. |
Aydogan K, Karadogan SK, Tunali S, Saricaoglu H. Narrowband ultraviolet B (311nm, TL01) phototherapy in chronic ordinary urticaria. Int J Dermatol 2012;58:98-103.
[Google Scholar]
|
| 17. |
Godse K. Methotrexate in autoimmune urticaria. Indian J Dermatol Venereol Leprol 2004;70:377.
[Google Scholar]
|
| 18. |
Perez A, Woods A, Grattan CE. Methotrexate: A useful steroid-sparing agent in recalcitrant chronic urticaria. Br J Dermatol 2010;162:191-4.
[Google Scholar]
|
| 19. |
Sagi L, Solomon M, Baum S, Lyakhovitsky A, Trau H, Barzilai A. Evidence for methotrexate as a useful treatment for steroid-dependant chronic urticaria. Acta Derm Venereol 2011;91:303-6.
[Google Scholar]
|
| 20. |
Zuberbier T, Asero R, Bindslev-Jensen C, Canonica GW, Church MK, Gime´nez-Arnau AM, et al. EAACI/GALEN/EDF/WAO guideline: Definition, classification and diagnosis of urticaria. Allergy 2009;64:1417-26.
[Google Scholar]
|
| 21. |
Konstantinou GN, Asero R, Maurer M, Sabroe RA, Schmid-Grandelmeier P, Grattan CE. EAACI/GALEN task force consensus report: The autologous serum skin test in urticaria. Allergy 2009;64:1256-68.
[Google Scholar]
|
| 22. |
Delong LK, Culler SD, Saini SS, Beck LA, Chen SC. Annual direct and indirect health care costs of chronic idiopathic urticaria: A cost analysis of 50 nonimmuno suppressed patients. Arch Dermatol 2008;144:35-9.
[Google Scholar]
|
| 23. |
Okubo Y, Shigoka Y, Yamazaki M, Tsuboi R. Double dose of cetirizine hydrochloride is effective for patients with urticaria resistant: A prospective, randomized, non-blinded, comparative clinical study and assessment of quality of life. J Dermatol Treat 2013;24:153-60.
[Google Scholar]
|
| 24. |
Zuberbier T, Asero R, Bindslev-Jansen C, Canonica GW, Church MK, Giminez-Arnau AM, et al. EAACI/GALEN/EDF/WAO guideline: Management of urticaria. Allergy 2009;64:1427-43.
[Google Scholar]
|
| 25. |
Maurer M, Rosén K, Hsieh HJ, Saini S, Grattan C, Giemez-Arnau A, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med 2013;368:924-35.
[Google Scholar]
|
| 26. |
Toubi E, Blant A, Kessel A, Golan TD. Low-dose cyclosporin A in the treatment of severe chronic idiopathic urticaria. Allergy 1997;52:312-6.
[Google Scholar]
|
Fulltext Views
2,493
PDF downloads
1,159





