Translate this page into:
Skin lightening agents - Use or abuse? - A retrospective analysis of the topical preparations used by melasma patients of darker skin types
Correspondence Address:
Rajat Kandhari
11, Munirka Marg, Vasant Vihar, New Delhi - 110 057
India
| How to cite this article: Kandhari R, Khunger N. Skin lightening agents - Use or abuse? - A retrospective analysis of the topical preparations used by melasma patients of darker skin types. Indian J Dermatol Venereol Leprol 2013;79:701-702 |
Sir,
Treatment with a triple combination (TC) agent appears to be the most clinically effective initial therapy for patients with melasma. [1] We carried out a retrograde analysis in 69 patients of melasma of the topical preparations used for more than 3 months. The objective of our study was to analyze the topical preparations used by patients of melasma, their mode of usage and their side effect profiles. The type of melasma and the skin type (Fitzpatrick classification) were noted in each patient. The preparations used were divided into 2 groups, use of a single agent or a combination of agents (combination creams or more than one agent). The usage of the agents, whether single or combination was further classified into 3 groups, as advised by a clinician, self-application or both. Each agent used was assessed on the basis of concentration used, mode of usage and type of usage, i.e., regular or intermittent. The 3 main agents assessed were hydroquinone (HQ), corticosteroids (CS) and tretinoin (Tr.). Patients, who had used combinations, were further classified into those who had used TCs containing HQ, CS, and Tr. (HQ + CS + Tr.) and those who had used a combination of hydroquinone and Tr. (HQ + Tr.), HQ and CS (HQ + CS) and CS and Tr. (CS + Tr.). The side-effect profile of each patient was assessed and a comparison was made between the side effects in the group of patients who used TC formulations versus those who had not used these formulations.
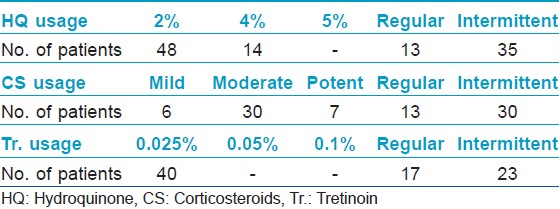
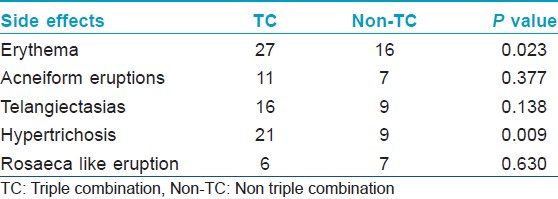
Majority of the study group (48 patients) belonged to Fitzpatrick skin type IV, and malar melasma was the most common morphological pattern noted in 38 patients. The combination most commonly used was a TC formulation which was used by 36 patients (52.17%). The other combinations included (HQ + CS) used by 10 patients (14.49%) and (HQ + Tr.) by four patients (5.79%). Usage of HQ as monotherapy was noted in 12 patients, whereas seven patients (10.1%) reported usage of topical CS as monotherapy. Tr. usage as monotherapy or (CS + Tr.) usage was not noted even in a single patient. As per the mode of usage, both self-application along with advised application was noted in a staggering 57.97% of patients, whereas self-application alone was seen in 28.98% of patients and application advised by a physician was noted in 13.04% of patients. TC formulations were used most commonly in an unsupervised manner. Further the concentrations used and the type of usage (regular or intermittent) was assessed. 2% HQ was used most commonly by 48 patients whereas 4% HQ was used by 14 out of the 69 patients. Thirty-Five patients reported usage of HQ in an intermittent fashion. Moderate to potent CS usage was more common than usage of mild CS. Usage of Tr. 0.025% was reported by 40 patients. Unsupervised, intermittent usage was far more common than regular usage [Table - 1]. The side effects experienced included erythema in 43 patients, epidermal atrophy in two patients, irritant contact dermatitis in one patient, acneiform eruptions in 18 patients, telangiectasias in 25 patients, hypertrichosis in 30 patients, rosacea-like eruption in 13 patients, confetti like depigmentation in eight patients [Figure - 1]a-f. None of the patients experienced an allergic reaction to the preparations used. We further compared the side effects experienced in patients who used TC agents versus those who had used other preparations. The Pearson′s Chi-square test was carried out to compare the two groups. Erythema and hypertrichosis in the group who used TC was significantly more as compared to the group who had not used the TC ( P < 0.05). The other side effects experienced by the group who used TC and those who had used other preparations did not show any statistically significant increase in either of the groups [Table - 2]. The lacunae of our analysis were a small sample size, lack of histological assessment and lack of control groups. To conclude we highlight the fact, that TC usage was highly rampant, whether advised by a clinician or as self-application. Most patients using these formulations used them for a longer duration than advised. Despite prolonged usage of TC formulations epidermal atrophy was not found to be a significant side effect, which may be due to the Tr. which is said to prevent the atrophy from occurring and renders these formulations "safe." [2] The TC users experienced significant erythema and hypertrichosis. Whether the erythema was due to the prolonged corticosteroid usage or the HQ in the formulations is unclear. The hypertrichosis due to the CS was not prevented by the Tr. present in the formulations. Even though various studies [3],[4] demonstrate the favorable side-effect profile of TC (HQ 4%, Tr. 0.05%, flucinolone acetonide 0.01%) used intermittently over long periods, we urge dermatologists to be cautious while prescribing these formulations and lay stress on the importance of a regular follow-up while doing so. The probable reason for unsupervised, self-usage of the TC is their ability to provide a rapid clearance of the melasma, which invariably results in the patient abruptly stopping the use of the formulation, thereby leading to a relapse and further application of the same formulation. [5] The Pigmentary Disorders Academy in 2006 suggested fixed TC agents as first line treatment for melasma patients [2] In our opinion, there is an urgent need for proper treatment guidelines for melasma management and agents such as HQ 2-4%, topical retinoids, alpha hydroxyl acids, azelaic acid, topical vitamin C and kojic acid in conjunction with regular photoprotection used under supervision may be safer alternatives to TC agents, even though they may be less efficacious and require a longer time to produce clinically visible improvement.
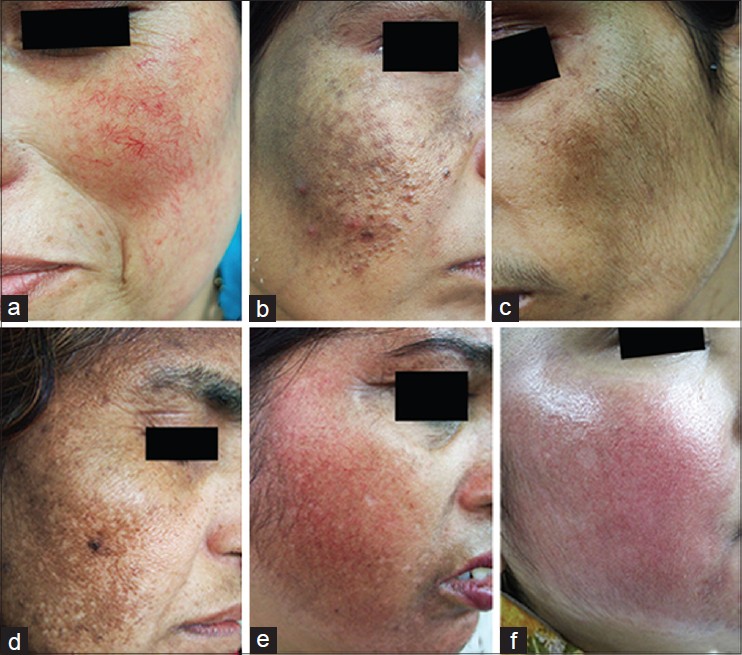 |
| Figure 1: Side effects due to the use of skin lightening agents; (a) Telangiectasias, (b) Acneiform eruptions, (c) Hypertrichosis, (d) Confetti-like depigmentation, (e) Rosacea-like eruption. (f) Erythema |
| 1. |
Sheth VM, Pandya AG. Melasma: A comprehensive update: Part II. J Am Acad Dermatol 2011;65:699-714.
[Google Scholar]
|
| 2. |
Rendon M, Berneburg M, Arellano I, Picardo M. Treatment of melasma. J Am Acad Dermatol 2006;54:S272-81.
[Google Scholar]
|
| 3. |
Torok HM, Jones T, Rich P, Smith S, Tschen E. Hydroquinone 4%, tretinoin 0.05%, fluocinolone acetonide 0.01%: A safe and efficacious 12-month treatment for melasma. Cutis 2005;75:57-62.
[Google Scholar]
|
| 4. |
Torok H, Taylor S, Baumann L, Jones T, Wieder J, Lowe N, et al. A large 12-month extension study of an 8-week trial to evaluate the safety and efficacy of triple combination (TC) cream in melasma patients previously treated with TC cream or one of its dyads. J Drugs Dermatol 2005;4:592-7.
[Google Scholar]
|
| 5. |
Majid I. Mometasone-based triple combination therapy in melasma: Is it really safe? Indian J Dermatol 2010;55:359-62.
[Google Scholar]
|
Fulltext Views
4,761
PDF downloads
870





