Translate this page into:
Role of insulin resistance and diet in acne
Correspondence Address:
Rashmi Kumari
Department of Dermatology and STD, JIPMER, Pondicherry - 605 006
India
| How to cite this article: Kumari R, Thappa DM. Role of insulin resistance and diet in acne. Indian J Dermatol Venereol Leprol 2013;79:291-299 |
Abstract
There is increasing evidence in support of the interplay of growth hormone (GH), insulin, and insulin-like growth factor-1 (IGF-1) signaling during puberty, which have a causal role in pathogenesis of acne by influencing adrenal and gonadal androgen metabolism. Milk consumption and hyperglycemic diets can induce insulin and IGF-1-mediated PI3K ⁄ Akt-activation inducing sebaceous lipogenesis, sebocyte, and keratinocyte proliferation, which can aggravate acne. Occurence of acne as part of various syndromes also provides evidence in favor of correlation between IGF-1 and acne.Introduction
From a phylogenetic relict or a kind of living skin fossil, the sebaceous gland turned to be considered the ′brain of the skin′ and an important cutaneous endocrine gland. [1] Ongoing research has revealed the role of androgens, follicular retention hyperkeratosis, increased sebaceous lipogenesis, increased colonization with P. acnes, inflammatory signaling, and regulatory neuropeptides involved in this multifactorial process, which may influence a hereditary predisposition to develop acne. [2]
There is increasing evidence in support of the interplay of growth hormone (GH), insulin, and insulin-like growth factor-1 (IGF-1) signaling during puberty, which may have a causal role in pathogenesis of acne by influencing adrenal and gonadal androgen metabolism. [3] Role of diet in acne was previously highly debated, but studies have shown that high milk consumption exacerbates acne by increasing the insulin/IGF-1 signaling. [4] Occurrence of acne as part of various syndromes associated with insulin resistance also provides evidence in favor of correlation between IGF-1 and acne. [5]
Understanding of Sebaceous Llipogenesis
The root of acne seems to lie at the juncture of hormone action and lipid metabolism in sebocyte differentiation. Acne will not develop without sebum, and sebum will not be produced without androgenic stimulation of sebocytes. Common inflammatory acne only occurs when androgens rise at puberty. Basic research suggests that the compensatory insulin excess independently aggravates the acne. [5],[6]
Growth Hormone and Insulin-Like Growth Factor-1 Axis
Growth hormone and IGF-1 are important in maintenance of epidermal homeostasis. Growth hormone is made in the anterior pituitary gland, and is released into the blood stream, and then stimulates the liver to produce IGF-1 [Figure - 1]. IGF-1 is a primary mediator of the effects of growth hormone (GH). IGF-1 then stimulates systemic body growth and has growth-promoting effects on almost every cell in the body.
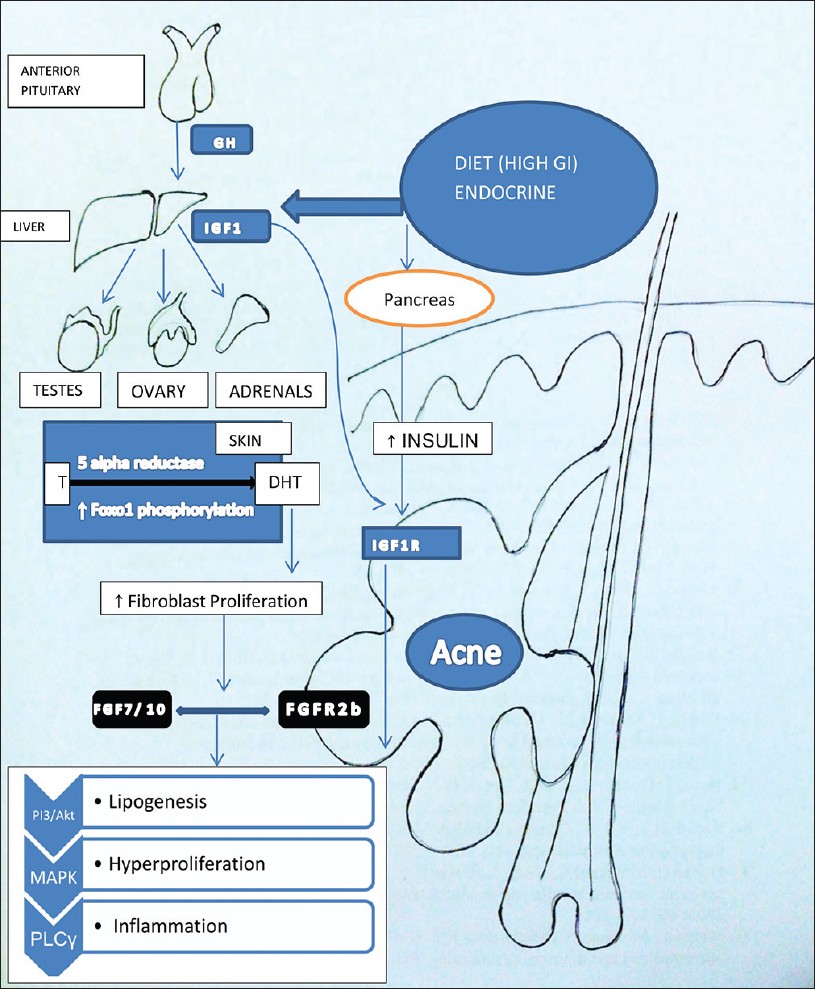 |
| Figure 1: Dietary factors increase the levels of IGF1 synthesized from liver. IGF1-mediated increased signaling of androgen receptor results in increased expression of FGF7 and FGF10, the ligands of FGFR2b signaling in keratinocyte. Both the IGF1R and FGFR2b activation results in a common downstream pathway via activation of PI3/Akt, MAPK, and phospholipase C activation with resultant increase in all the three factors responsible for acne. (T- in the figure stands for testosterone) |
Insulin-like growth factor (IGF) system includes IGF-1, IGF-2, and proinsulin, three IGF receptors (IGF-1 receptor, IGF-2 receptor, insulin receptor) and six IGF binding proteins (IGFBP1-IGFBP6). These have different roles in cell proliferation, protein synthesis, carbohydrate homeostasis, and bone metabolism. IGF-1R is a single transmembrane domain receptor harboring intrinsic tyrosine kinase activity, which can be activated by IGF1 and high insulin concentrations. [7],[8]
Hyperinsulinemia and IGF1 Levels Stimulate Sebaceous Lipogenesis
Recent studies have shown that elevated levels of serum insulin-like growth factor-I (IGF-I) correlate with overproduction of sebum and acne. Recently, in a study, IGF-I (CA) 19 polymorphism has been shown to contribute to a predisposition to acne in Turkish patients. [9] IGF-1 and insulin stimulate lipogenesis of sebaceous glands, probably by induction of sterol response element-binding protein1 (SREBP1). [10] Insulin-like growth factor-I (IGF-I) is synthesized in the skin, mainly by dermal fibroblasts and melanocytes and also possibly by keratinocytes of the stratum granulosum. The expression of IGF1R mRNA was found to be the strongest in basal cells of sebaceous glands and immature sebocytes, whereas IGF1R-protein expression was uniform and intense in all regions of the gland. This pattern of expression suggests a role for IGF-1 as a sebaceous mitogen and morphogen. [6] Insulin is a mitogen when it binds to insulin receptor-A (IR-A) or IGF-1R. For its metabolic actions and in glucose metabolism, it binds to insulin receptor (IR-B). [10]
Even though acne is considered an androgen-dependent disease, occurence of acne doesn′t correlate with plasma androgen levels. Increased serum levels of IGF-1 have been observed in adult women and men with acne, giving rise to the possibility of the role of GH, hyperinsulinemia, and IGF1 in acne. [11],[12] A positive correlation between the mean facial sebum excretion rate and serum IGF-1 levels has been demonstrated in post-adolescent acne patients. [13] Cappel, et al. demonstrated that IGF-1 levels correlate with severity of acne in women. [12]
IGF1 and its Role of Increased Androgen Synthesis
Skin is the largest endocrine organ of the body. Androgens play an essential role in increasing the size of sebaceous glands and stimulating sebum production as well as in stimulating keratinocyte proliferation in the ductus seboglandularis and the acroinfundibulum. [14] Conditions of androgen excess or hyperandrogenism are associated with increased sebum production and the development of severe acne. Acne-prone skin exhibits a higher androgen receptor density and higher 5α-reductase activity than uninvolved skin. Conversely, anti-androgens reduce the synthesis of sebaceous lipids and improve acne, whereas androgen-insensitive subjects who lack functional androgen receptors do not produce sebum and do not develop acne. [15]
Hyperinsulinemia promotes acne by its well-known androgenic stimulation to adrenals, testes as well as the ovaries [Figure - 1].
Normal puberty is characterized by a state of transient insulin resistance associated with an increase in gonadal sex steroid production and adrenal androgens. [16] IGF-1 enhances the sensitivity of the adrenal for ACTH and induces the expression and activity of key enzymes of adrenal androgen biosynthesis like dehydroepiandrosterone sulfate (DHEAS). [16],[17] In healthy prepubertal girls, as well as in prepubertal girls with premature adrenarche, a positive correlation between IGF-1 and DHEAS serum levels has been reported. [18]
Insulin resistance has been associated with polycystic ovarian syndrome not only in obese females but also in lean females with polycystic ovarian syndrome (PCOS) with upper body fat distribution. Insulin receptors are present on the ovaries, and it has been shown in vitro that insulin increases the luteinizing hormone (LH)-dependent androgen secretion of thecal cells and that insulin can directly stimulate androgen production by ovarian stroma cells. After the rise of luteinizing hormone (LH), there is a significant increase in IGF-1 and progesterone in the dominant follicle. [19] IGF-1 exerts stimulatory effects on estrogen synthesis of granulosa cells and ovarian steroidogenesis by increasing the efficacy of LH on interstitial theca-cells. [19]
In males, IGF-system is of importance for Leydig-cell differentiation, mitogenesis, anti-apoptosis, and androgen biosynthesis. [20] Testicular levels of IGF-1 increases during puberty and coincides with increased production of testosterone. IGF-1, in addition to LH, stimulates the proliferation of Leydig-cell precursors and is an essential local mediator of testicular DNA synthesis and steroidogenesis.
IGF-1 Potentiates Peripheral Androgenism Via Foxo1 Phosphorylation
Acne occurs in adolescents at a time when GH is maximally secreted and levels of IGF1 are highest. [6] Melnik proposed that the peripheral amplification of androgen signaling by IGF-1 occurs in two ways [Figure - 1]. [3] One is by increasing the 5-alpha reductase activity with increased conversion of testosterone to dihydrotestosterone in skin [Table - 1]. Another mechanism of this potentiation of androgens is by alleviating the androgen receptor (AR) repression resulting in AR gain-of-function. [21] Melnik proposed that acne pathogenesis is related to nuclear transcription factor forkhead box (Fox 01) deficiency resulting from export of nuclear Fox 01 to cytoplasm [Figure - 1]. In the nucleus, AR binds to the AR repressive protein Fox 01. [22] IGF-1 as well as insulin activates PI3K, which leads to Akt-mediated Fox 01 phosphorylation. As a result of this phosphorylation, Fox 01 leaves the AR and translocates from the nucleus into the cytoplasm and hence potentiates the action of androgen receptor. [2],[3]
FGFR2b and Igf1r Interactions in Sebocyte Differentiation and Lipogenesis
FGFR′s are a family of tyrosine kinase receptors expressed on the suprabasal cells of sebaceous glands, whereas IGF1R is expressed on basal cells. [23] They regulate cellular pathways involved in proliferation and differentiation of sebocytes and keratinocytes by activating MAPK and PI3K/Akt signaling pathways.
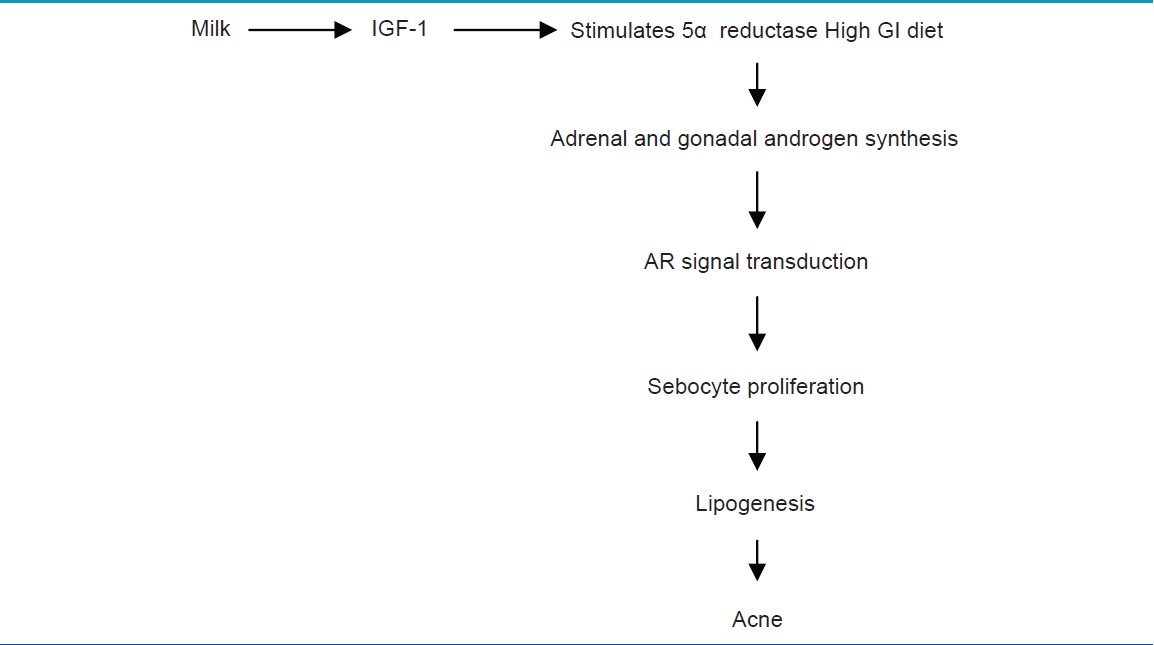
Comedogenesis is considered to be a process of increased keratinocyte proliferation as well as exaggerated keratinocyte differentiation (hyperkeratinization). IGF1R primarily regulates cellular proliferation and to a lesser extent differentiation, whereas FGFR2b is predominantly involved in cellular differentiation [Figure - 1]. [23] IGF-1- mediated increased signaling of androgen receptor increases the perifollicular fibroblast proliferation and increased expression of FGF7 and FGF10, the ligands of FGFR2b. Increased expression on these receptors and further signaling resulting in acne is shown in [Table - 2] . [3]
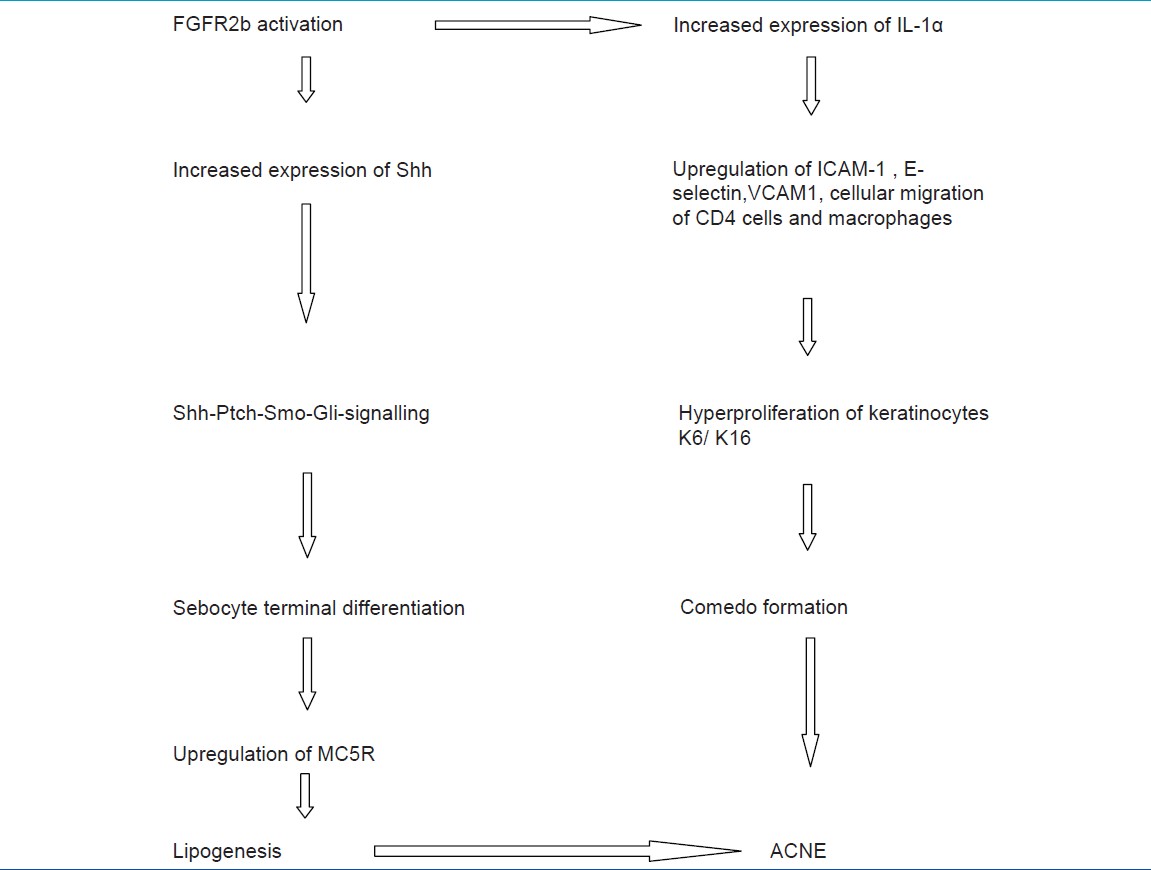
With the onset of puberty, there is increase in plasma androgen levels, which stimulate the perifollicular stroma of infundibulum and sebaceous glands. Increased expression of FGF7 and FGF10 and through paracrine effect bind on FGFR2b on sebocytes and infundibular keratinocytes. This activation of FGFR2b results in increased expression of sonic hedgehog pathway (Shh), signaling, and increased expression of IL-1α. [24]
Aggravation of Acne By Dietary Modification Via Insulin ⁄ Igf-1 Signaling
Is it a myth or a reality? For long, the association of diet with acne had been dismissed, and most dermatologists do not advise any dietary recommendations to patients with acne. But, when Cordain et al. published his study in 1200 Kitavan islanders of Papua New Guinea and 115 hunter-gatherers of Paraguay who do not consume dairy products and have low glycemic diets and found an absence of acne in this population, this association was again brought to light. But it was not clear whether only diet was responsible or genetic factors were also involved for absence of acne in this population. [25]
Hyperinsulinemia, Insulin Resistance, IGF1, and IGFBP3
Compelling evidence exists that high glycemic load diets exacerbate acne by increasing the levels of IGF1. Smith et al. demonstrated that low glycemic load diet for 12 weeks decreased serum IGF-1 levels and significantly improved acne. [26] Dietary intervention increases the nuclear content of Fox 01, thereby normalizing increased transcription of genes involved in acne.
Eating high glycemic index foods causes hyperglycemia. The pancreas responds to hyperglycemia by releasing large quantities of insulin to bring down the blood sugar levels. Large quantities of insulin causes blood sugar levels to fall down rapidly. Too low blood sugar levels trigger another stress response in adrenal glands. The adrenal glands release androgens that signal the liver to release some of its glycogen storages to raise the blood sugar to normal. Incidentally, low blood sugar levels also trigger serious craving for food. With these cravings, the tendency is to again eat food with high glycemic index and the cycle continues chronically.
The more the pancreas releases insulin, the less effective it becomes as a result of reduced sensitivity of the cells to insulin. This is called insulin resistance or reduced insulin sensitivity. To counter this effect, the pancreas has to release increased secretion of insulin from pancreas [27] [Figure - 1]. Chronic and acute hyperinsulinemia initiates the hormone cascade that favors tissue growth by stimulating increased levels of free IGF1 and reducing levels of IGF binding protein3. [28] Because free IGF1 is a potent mitogen for virtually all body tissues, it promotes acne via hyperkeratinization. Reduction in IGFBP3 levels after hyperinsulinemia or after ingestion of high glycemic food also makes more free IGF1 available and upregulates cell proliferation. IGFBP3 is also a ligand for RXR α nuclear retinoid receptor and enhances RXR homodimer-mediated signaling. Low IGFBP3 may reduce effectiveness of natural retinoids present in skin to activate genes that would limit follicular cell proliferation. Hyperinsulinemia also increases the number of EGF′s and TGFβ, which elevates plasma non-esterified fatty acids. These fatty acids decrease the levels of IGFBP3 and increase in IGF1 levels.
Various Studies on Relationship of Diet to Acne
Chiu et al. [28] in a study among university students showed a worsening perceived diet quality was positively associated with acne. But, this study included a small sample size and use of a tool that has not been validated for measuring diet quality. Adebamowo et al. in a prospective cohort study demonstrated a correlation between milk consumption and acne, but this may be caused by hormones and bioactive molecules present in skimmed milk. [29] Another study by the same author later established that it is the hydrophilic protein fraction in cow′s milk and not the lipophilic androgenic steroids enriched in milk fat, which increases insulin / IGF-1 signaling with milk-induced aggravation of acne. [30] This study was also a self-reported questionnaire-based study. This finding requires further exploration as it is still possible that the androgens derived from the pregnant cow′s body secreted in the milk are responsible for the increased expression of hormonal activity during puberty. Milk is a rich source of active IGF-1 and IGF-2 even after pasteurization and homogenization. [31] High milk consumption is associated with a 10-20% increase in circulating IGF-1 levels among adults and a 20- 30% increase among children. [32],[33] Milk and dairy products increase IGF-1 levels more than other dietary sources of protein such as meat. [34],[35] After milk consumption for 1 month, children have shown to have a higher mean plasma level of IGF-1, higher IGF-1 / IGFBP-3, and GH levels. [36] In a 1-week intervention study of 24 prepubertal 8-year-old boys, the effect of daily intake of 53 g of either lean meat or skim milk (1.5 liter per day) was studied with regard to insulin and IGF-1 responses. There was a significant increase in insulin and IGF1 in the skim milk group but no similar increase in the meat group. [37]
The major protein fraction of cow′s milk is casein (80%), and the remaining 20% are whey proteins. The insulinotropic component of milk resides predominantly within the whey fraction, whereas casein has a stronger IGF-1 stimulating effect than does whey. Inclusion of milk and hyperglycemic foods in diet may have potentiating effects on serum insulin and IGF-1 levels, thereby promoting the development of acne. [38]
Androgen abuse in form of recombinant GH, insulin, and insulinotropic whey protein concentrates are advertised and used by many fitness centers as it would increase IGF1 levels. [39]
Stress and Insulin Resistance
One of the downstream targets of IGF-1 signaling is to repress stress resistance proteins including antioxidant enzymes like superoxide dismutase and heat shock proteins. So an increase in IGF signaling may decrease the expression of stress resistance genes and exacerbate cellular inflammation increasing acne. The link between caloric restriction and IGF signaling may be that a reduction in food intake reduces the expression of IGF-1, increasing the expression of stress resistance proteins and hence may benefit those with severe acne. [40]
Smoking and Insulin Resistance
Hyperinsulinemia, dyslipidemia, and exaggerated adrenal androgen response to ACTH have been observed in male smokers. [41] Smoking may inhibit the adrenal 21-hydroxylase resulting in an increase in the production of adrenal androgens, which contribute to the insulin resistance in smokers and those with hidradenitis suppurativa. [42]
Endocrine Disorders with Increased Insulin- and IGF-1 Serum Levels and Acne
Laron syndrome is characterized by GH resistance, molecular defects of the GHR, or post-receptor pathways leading to inability to synthesize IGF-1. During IGF-1 treatment of six female patients with Laron syndrome, four developed oligo / amenorrhea and acne associated with significant elevations in serum testosterone and androstenedione. [43] Reduction of the IGF-1 dose or interruption of IGF-1 treatment normalized androgen levels and resulted in resolution of acne and oligomenorrhea. This provides an indirect proof of the role of IGF1 in development of acne. Acne occurs as part of various syndromes such as PCOS (polycystic ovary syndrome), [44] HAIRAN syndrome (hyperandrogenism, insulin resistance, and acanthosis nigricans), [45] congenital adrenal hyperplasia (androgenetic alopecia, hirsutism, and acne), [46]
Endocrine Disrupting Chemicals and Acne
An endocrine-disrupting substance (EDS) is a compound, either natural or synthetic, which through environmental or inappropriate developmental exposures alters the hormonal and homeostatic systems. Because of the shared properties of the chemicals and the similarities of the receptors and enzymes involved in the synthesis, release, and degradation of hormones, no endocrine system is immune to endocrine disrupting chemicals including the sebaceous glands. [47] Milk and diet-related chemicals reaching the body may act as EDS and cause acne. Effects of endocrine disrupting chemicals may be transmitted to further generations through germline epigenetic modifications or from continued exposure of offspring to the environmental insult. Natural chemicals found in human and animal food (e.g., phytoestrogens, including genistein and coumestrol) can also act as endocrine disruptors. These substances, whereas generally thought to have relatively low binding affinity to estrogen receptors, are widely consumed and are components of infant formula. Therefore, the potential for endocrine disruption by phytoestrogens needs to be considered. Polychlorinated biphenyls (PCBs), resulting in chloracne in form of Yusho (rice oil disease), observed in Japan as an example of environmental contaminant in day-to-day food resulting in acne.
Insulin and IGF-1 Signaling in Acne Treatment
Retinoids not only suppress FGFR2-signaling but also have opposing effects on IGF1R and androgen receptor signal transduction. [48] IGF-1 is an inducer of 5α-reductase activity, whereas isotretinoin significantly reduces the activity of 5α-reductase in the skin of acne patients. [49] Isotretinoin induces apoptosis and cell cycle arrest in human SEB-1 sebocytes. [50] IGF-1 and insulin activate Fox 01 phosphorylation, thereby augmenting androgen receptor signaling. [21] Whereas oral isotretinoin treatment decreased the androgen receptor binding capacity constant in the skin of acne patients by a factor of 2.6. [51] Retinoids also downregulate melanocortin receptor expression, which causes sebocyte differentiation and lipogenesis. [52]
Metformin treatment has ′counter-regulatory′ action to the puberty-induced shift of the insulin / IGF-1 axis to higher levels. Patients with PCOS on metformin treatment showed a decrease in elevated serum IGF-1 and androgen levels. [53] Metformin treatment of girls with precocious pubarche prevented the onset of early puberty by 0.4 years and significantly decreased serum levels of IGF-1, fasting insulin, DHEAS, and testosterone. [54]
Conclusion
Growth hormone (GH), insulin, and insulin-like growth factor-1 (IGF-1) signaling during puberty may have a causal role in pathogenesis of acne by influencing adrenal and gonadal androgen metabolism. Recent studies have reopened the debate on association of diet with acne. Certain food with high glycemic index and milk may exacerbate acne by increasing the insulin/IGF-1 signaling pathway. But, this fact still needs confirmation due to lack of randomized trials and difficulty in maintaining subjects on an exclusive diet for long periods. Occurence of acne as part of various syndromes and drugs useful in treatment of acne also utilize the growth hormone, and IGF1 axis may be proof in favor of correlation between IGF-1 and acne.
| 1. |
Zouboulis CC, Baron JM, Böhm M, Kippenberger S, Kurzen H, Reichrath J, et al. Frontiers in sebaceous gland biology and pathology. Exp Dermatol 2008:17;542-51.
[Google Scholar]
|
| 2. |
Melnik BC. FoxO1-the key for the pathogenesis and therapy of acne? J Dtsch Dermatol Ges. 2010:8;105-14.
[Google Scholar]
|
| 3. |
Melnik BC, Schimtz G. Role of insulin, insulin like growth factor-1, hyperglycemic food and milk components in pathogenesis of acne vulgaris. Exp Dermatol 2009;18:833-41.
[Google Scholar]
|
| 4. |
Smith R, Mann N, Braue A, Ma kelainen H, Varigos GA. The effect of a high protein, low glycemic load diet versus a conventional, high glycemic load diet on biochemical parameters associated with acne vulgaris. J Am Acad Dermatol 2007:57:247-56.
[Google Scholar]
|
| 5. |
Chen W, Obermayer-Pietsch B, Hong JB, Melnik BC, Yamasaki O, Dessinioti C, et al. Acne-associated syndro mes: Models for better understanding of acne pathogenesis. J Eur Acad Dermatol Venereol 2011;25:637-46.
[Google Scholar]
|
| 6. |
Thiboutot D. Regulation of human sebaceous glands. Invest Dermatol 2004;123:1-12.
[Google Scholar]
|
| 7. |
Edmondson SR, Thumiger SP, Werther GA, Wraight CJ. Epidermal homeostasis: The role of growth hormone and insulin-like growth factor systems. Endocr Rev 2003:24:737-64.
[Google Scholar]
|
| 8. |
Denley A, Cosgrove LJ, Booker GW, Wallace JC, Forbes BE. Molecular interactions of the IGF system. Cytokine Growth Factor Rev 2005:16:421-39.
[Google Scholar]
|
| 9. |
Tasli L, Turgut S, Kacar N, Ayada C, Coban M, Akcilar R, et al. Insulin-like growth factor-I gene polymorphism in acne vulgaris. J Eur Acad Dermatol Venereol 2011 Oct 10 [Epub ahead of print].
[Google Scholar]
|
| 10. |
Deplewski D, Rosenfield RL. Growth hormone and insulin-like growth factors have different effects on sebaceous cell growth and differentiation. Endocrinology 1999:140:4089-94.
[Google Scholar]
|
| 11. |
Deplewski D, Rosenfield RL. Role of hormones in pilosebaceous unit development. Endocr Rev 2000:21:363-92.
[Google Scholar]
|
| 12. |
Cappel M, Mauger D, Thiboutot D. Correlation between serum levels of insulin-like growth factor 1, dehydroepiandrosterone sulfate, and dihydrotestosterone and acne lesion counts in adult women. Arch Dermatol 2005:141:333-8.
[Google Scholar]
|
| 13. |
Vora S, Ovhal A, Jerajani H, Nair N, Chakraborty A. Correlation of facial sebum to serum insulin-like growth factor-1 in patients with acne. Br J Dermatol 2008:159:979-95.
[Google Scholar]
|
| 14. |
Zouboulis CC. The human skin as a hormone target and an endocrine gland. Hormones 2004;3:9-26.
[Google Scholar]
|
| 15. |
Imperato-McGinley J, Gautier T, Cai LQ, Yee B, Epstein J, Pochi P. The androgen control of sebum production. Studies of subjects with dihydrotestosterone deficiency and complete androgen insensitivity. J Clin Endocrinol Metab 1993;76:524-8.
[Google Scholar]
|
| 16. |
Belgorosky A, Baquedano MS, Guerico G, Rivarola MA. Adrenarche: Postnatal adrenal zonation and hormonal and metabolic regulation. Horm Res 2008;70:257-67.
[Google Scholar]
|
| 17. |
Mesiano S, Katz SL, Lee JY, Jaffe RB. Insulin-like growth factors augment steroid production and expression of steroidogeneic enzymes in human fetal adrenal cortical cells: Implications for adrenal androgen regulation. J Clin Endocrinol Metabol 1997;82:1390-6.
[Google Scholar]
|
| 18. |
Guerico G, Rivarola MA, Chaler E, Maceiras M, Belgorosky A. Relationship between the growth hormone / insulin-like growth factor-I axis, insulin sensitivity, and adrenal androgens in normal prepubertal and pubertal girls. J Clin Endocrinol Metab 2003;88:1389-93.
[Google Scholar]
|
| 19. |
Cara JF. Insulin-like growth factors, insulin-like growth factor binding proteins and ovarian androgen production. Horm Res 1994;42:49-54.
[Google Scholar]
|
| 20. |
Colo×n E, Svechnikov KV, Carlsson-Skwirut C, Bang P, Soder O. Stimulation of steroidogenesis in immature rat leydig cells evoked by interleukin-1a is potentiated by growth hormone and insulin-like growth factors. Endocrinology 2005;146:221-30.
[Google Scholar]
|
| 21. |
Fan W, Yanase T, Morinaga H, Okabe T, Nomura M, Daitoku H, et al. Insulin-like growth factor 1 / insulin signalling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J Biol Chem 2007;282:7329-38.
[Google Scholar]
|
| 22. |
Melnik B, Schmitz G. FGFR2 signaling and the pathogenesis of acne. J Dtsch Dermatol Ges 2008;6:721-8.
[Google Scholar]
|
| 23. |
Kaushansky A, Gordus A, Chang B, Rush J, MacBeath G. A quantitative study of the recruitment potential of all intracellular tyrosine residues on EGFR, FGFR1 and IGF1R. Mol Biosyst 2008;4:643-53.
[Google Scholar]
|
| 24. |
Melnik BC. Role of FGFR2-signaling in the pathogenesis of acne. Dermatoendocrinol 2009;1:141-56.
[Google Scholar]
|
| 25. |
Cordain L, Lindeberg S, Hurtado M, Hill K, Eaton SB, Brand-Miller J. Acne vulgaris. A disease of Western civilization. Arch Dermatol 2002;138:1584-90.
[Google Scholar]
|
| 26. |
Smith R, Mann N, Makelainen H, Roper J, Braue A, Varigos G. A pilot study to determine the short-term effects of a low glycemic load diet on hormonal markers of acne: A nonrandomized, parallel, controlled feeding trial. Mol Nutr Food Res 2008;52:718-26.
[Google Scholar]
|
| 27. |
Berra B, Rizzo AM. Glycemic index, glycemic load: New evidence for a link with acne J Am Coll Nutr 2009;28:450S-4.
[Google Scholar]
|
| 28. |
Chiu A, Chon SY, Kimball AB. The response of skin disease to stress: Changes in the severity of acne vulgaris as affected by examination stress. Arch Dermatol 2003;139:897-900.
[Google Scholar]
|
| 29. |
Adebamowo CA, Spiegelman D, Berkey CS, Danby FW, Rockett HH, Colditz GA, et al. Milk consumption and acne in adolescent girls. Dermatol Online J 2006;12:1-12.
[Google Scholar]
|
| 30. |
Adebamowo CA, Spiegelman D, Berkey CS, Danby FW, Rockett HH, Colditz GA, et al. Milk consumption and acne in teenaged boys. J Am Acad Dermatol 2008;58:787-93.
[Google Scholar]
|
| 31. |
Blum JW, Baumrucker CR. Insulin-like growth factors (IGFs), IGF binding proteins, and other endocrine factors in milk: Role in the newborn. In: Bosze Z, editors. Vol. 606. Bioactive Components of Milk. Advances in Experimental Medicine and Biology. New York: Springer; 2008. p. 397-422.
[Google Scholar]
|
| 32. |
Hoppe C, Udam TR, Lauritzen L, Mølgaard C, Juul A, Michaelsen KF. Animal protein intake, serum insulin-like growth factor I, and growth in healthy 2.5-yold Danish children. Am J Clin Nutr 2004;80:447-52.
[Google Scholar]
|
| 33. |
Rogers IS, Gunnell D, Emmett PM, Glynn LR, Dunger DB, Holly JM. Cross-sectional associations of diet and insulin-like growth factor levels in 7- to 8-year-old children. Cancer Epidemiol Biomarkers Prev 2005;14:204-12.
[Google Scholar]
|
| 34. |
Esterle L, Sabatier JP, Guillon-Metz F, Walrant-Debray O, Guaydier-Souquières G, Jehan F, et al. Milk, rather than other foods, is associated with vertebral bone mass and circulating IGF-1 in female adolescents. Osteoporos Int 2009;20:567-75.
[Google Scholar]
|
| 35. |
Norat T, Dossus L, Rinaldi S, Overvad K, Grønbaek H, Tjønneland A, et al. Diet, serum insulin-like growth factor-I and IGF-binding protein-3 in European women. Eur J Clin Nutr 2007;61:91-8.
[Google Scholar]
|
| 36. |
Rich-Edwards JW, Ganmaa D, Pollak MN, Nakamoto EK, Kleinman K, Tserendolgor U, et al. Milk consumption and the prepubertal somatotropic axis. Nutr J 2007;6:28.
[Google Scholar]
|
| 37. |
Hoppe C, Molgaard C, Vaag A, Barkholt V, Michaelsen KF. High intakes of milk, but not meat, increase insulin and insulin resistance in 8-year-old boys. Eur J Clin Nutr 2005;59:393-8.
[Google Scholar]
|
| 38. |
Hoppe C, Molgaard C, Michaelsen K F. Cow's milk and linear growth in industrialized and developing countries. Annu Rev Nutr 2006;26:131-73.
[Google Scholar]
|
| 39. |
Melnik B, Jansen T, Grabbe S. Abuse of anabolic-androgenic steroids and bodybuilding acne: An underestimated health problem. J Dtsch Dermatol Ges 2007;5:110-7.
[Google Scholar]
|
| 40. |
Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature 2003;421:182-7.
[Google Scholar]
|
| 41. |
Hautanen A, Adlercreutz H. Hyperinsulinaemia, dyslipidaemia and exaggerated adrenal androgen response to adrenocorticotropin in male smokers. Diabetologia 1993;36:1275-81.
[Google Scholar]
|
| 42. |
Revuz JE, Canoui-Poitrine F, Wolkenstein P, Viallette C, Gabison G, Pouget F, et al. Prevalence and factors associated with hidradenitis suppurativa: Results from two case-control studies. J Am Acad Dermatol 2008;59:596-601.
[Google Scholar]
|
| 43. |
Melnik BC, John SM, Schmitz G. Over-stimulation of insulin/IGF-1 signaling by western diet may promote diseases of civilization: Lessons learnt from laron syndrome. Nutr Metab 2011;8:41.
[Google Scholar]
|
| 44. |
Melnik B, Vakilzadeh F, Aslanidis C, Schmitz G. Unilateral segmental acneiform nevus-a model disorder towards understanding FGFR2 function in acne? Br J Dermatol 2008;158:1397-9.
[Google Scholar]
|
| 45. |
Elmer KB, George RM. HAIR-AN syndrome: A multisystem challenge. Am Fam Physician 2001;63:2385-90.
[Google Scholar]
|
| 46. |
Dessinioti C, Katsambas AD. Congenital adrenal hyperplasia. Dermato-endocrinol 2009;1:87-91.
[Google Scholar]
|
| 47. |
Kandaraki E, Chatzigeorgiou A, Livadas S, Palioura E, Economou F, Koutsilieris M, et al. Endocrine disruptors and polycystic ovary syndrome (PCOS): Elevated serum levels of bisphenol A in women with PCOS. J Clin Endocrinol Metab 2011;96:E480-4.
[Google Scholar]
|
| 48. |
Melnik B, Schmitz G, Zouboulis CC. Anti-acne agents attenuate FGFR2 signal transduction in acne. J Invest Dermatol 2009;129:1868-77.
[Google Scholar]
|
| 49. |
Horton R, Pasupuletti V, Antonipillai I. Androgen induction of 5a-reductase may be mediated via insulin-like growth factor-I. Endocrinology 1993:133:447-51.
[Google Scholar]
|
| 50. |
Nelson AM, Gilliland KL, Cong Z, Thiboutot DM. 13-cis retinoic acid induces apoptosis and cell cycle arrest in human SEB-1 sebocytes. J Invest Dermatol 2006:126:2178-89.
[Google Scholar]
|
| 51. |
Boudou P, Soliman H, Chivot M, Villette JM, Vexiau P, Belanger A, et al. Effect of oral isotretinoin treatment on skin androgen receptor levels in male acneic patients. J Clin Endocinol Metab 1995:80:1158-61.
[Google Scholar]
|
| 52. |
Zhang L, Li WH, Anthonavage M, Pappas A, Rossetti D, Cavender D, et al. Melanocortin-5 receptor and sebogenesis. Eur J Pharmacol 2011;660:202-6.
[Google Scholar]
|
| 53. |
Berker B, Emral R, Demirel C, Corapcioglu D, Unlu C, Kose K. Increased insulin-like growth factor-I levels in women with polycystic ovary syndrome, and beneficial effects of metformin therapy. Gynecol Endocrinol 2004:19:125-33.
[Google Scholar]
|
| 54. |
Ibanez L, Ong K, Valls C, Marcos MV, Dunger DB, de Zegher F. Metformin treatment to prevent early puberty in girls with precocious pubarche. J Clin Endocrinol Metab 2006:91:2888-91.
[Google Scholar]
|
Fulltext Views
16,530
PDF downloads
1,949





