Translate this page into:
Severe cutaneous adverse drug reaction to leflunomide: A report of five cases
2 Department of Clinical Pharmacy, J. S. S. Medical College Hospital, Ramanuja Road, Mysore - 570 004, Karnataka, India
Correspondence Address:
Veeranna Shastri
Department of Skin and STD, J. S. S. Hospital, Ramanuja Road, Mysore - 570 004, Karnataka
India
| How to cite this article: Shastri V, Betkerur J, Kushalappa P A, Savita T G, Parthasarathi G. Severe cutaneous adverse drug reaction to leflunomide: A report of five cases. Indian J Dermatol Venereol Leprol 2006;72:286-289 |
Abstract
Medications used to treat human ailments are known to cause cutaneous reactions which may vary in their severity. Leflunomide, an immunomodulating agent recently introduced to treat rheumatoid arthritis, is reported to cause severe cutaneous reactions. We are reporting five such cases. All our patients were started on leflunomide for rheumatoid arthritis, 4-6 weeks before the onset of cutaneous reaction and were admitted to the hospital with the common complaints of fever, skin rash and generalized weakness. All of them had characteristic pattern of events such as delayed onset of reaction, widespread and long lasting skin rash and internal organ involvement. These features suggest a possibility of drug hypersensitivity syndrome to leflunomide. Careful dosing and periodic monitoring of patients treated with leflunomide for possible adverse drug reaction is recommended.


 |
 |
 |
 |
 |
 |
Introduction
Cutaneous adverse drug reactions (CADRs) are the most prominently seen ADRs. The clinical spectrum of CADRs ranges from pruritus to severe life-threatening reactions like erythema multiforme (EM), Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN) and drug hypersensitivity syndrome (DHS). Many drugs including ciprofloxacin, carbamazepine, dapsone, isoniazid, clindamycin, diclofenac, rifampicin and zidovudine are known to cause severe cutaneous reactions.
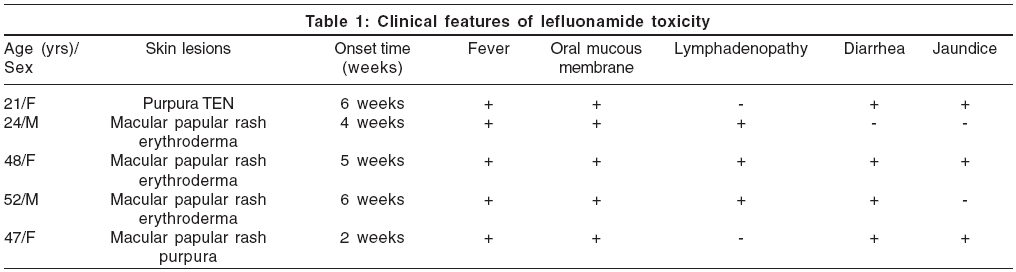
Leflunomide is a new immunomodulating agent and disease-modifying antirheumatic drug with anti-inflammatory and immunosuppressive activity, used in the treatment of active rheumatoid arthritis. It has been reported to cause various CADRs including SJS, TEN and EM, in less than 1% of the patient population.[1] We report five cases of severe CADR induced by leflunomide [Table - 1].
Case Reports
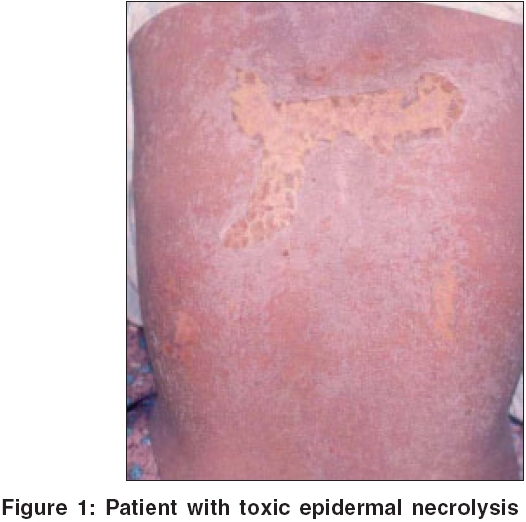
Case 1 A 21-year-old female patient was admitted to the hospital with history of fever, jaundice and pruritic skin rashes for the last 20 days. Six weeks prior to the admission to the hospital, she was treated with leflunomide (100 mg for 3 days, followed by 20 mg once daily) and rofecoxib (25 mg once daily) for arthralgia. On examination, she had icterus, generalized macular and papular rash and a few purpuric lesions over the legs. She also had right-sided minimal pleural effusion and hepatomegaly. Lab investigations revealed elevated WBC count and LFT [Table - 2]. After initial improvement with steroids and other supportive treatment, her condition worsened and she developed toxic epidermal necrolysis [Figure - 1], with a fatal outcome after a week.
Case 2 A 24-year-old male patient presented with pruritic skin rashes, fever, loss of appetite, generalized weakness and swelling of lips for the last 15 days. Four weeks prior to the symptoms, all other medications were withdrawn and he was started on the standard dosing regimen of leflunomide for arthritis. On examination, he was febrile with tachycardia. He had diffuse erythema over the trunk, multiple tiny papules and few pustules over the trunk and extremities [Figure - 2]. Crusting of lips, edema of hands and feet, congestion of eyes and left axillary lymphadenopathy were observed. Lab investigations revealed elevated WBC count and eosinophilia [Table - 2]. The patient′s condition improved with systemic steroids and supportive medications.
Case 3 A 50-year-old female patient presented with pruritic skin rashes and fever for the past 10 days. She was started on oral leflunomide for her arthritis, a month earlier. On examination, she had generalized erythematous macules and papules, edema of the face and enlarged posterior cervical lymph nodes [Figure - 3]. She developed jaundice, abdominal pain, diarrhea and vomiting during the hospital stay. Lab investigations revealed elevated LFT′s, WBC count and ESR [Table - 2]. The patient was treated with systemic steroids and supportive medications as required, but however, she developed exfoliative dermatitis. The skin biopsy showed features of interface dermatitis.
Case 4 A 52 year old male patient presented with pruritic skin rashes, fever, diarrhea, nausea and abdominal pain since 5 days. The patient was a known case of rheumatoid arthritis (RA), for which he was started on leflunomide, 6 weeks prior to the onset of the above symptoms. On examination, generalized erythematous papules, macules, patches with scaling and erosions on proximal extremities, were observed. Congestion of conjunctiva and oral mucous membrane was present. Right axillary lymph node was enlarged and non-tender. Lab investigations revealed elevated WBC count and ESR [Table - 2]. Skin biopsy showed features of interface dermatitis. The patient′s condition improved with systemic steroids and supportive medications. He developed exfoliative dermatitis during hospital stay.
Case 5
A 47-year-old female patient, a known case of rheumatoid arthritis, was started on leflunomide to control her worsening arthritis. Two weeks after starting the patient on a loading dose of leflunomide, the patient developed vomiting, followed by rashes all over the body. Rashes started as erythematous papules and later became generalized. Lab investigations revealed low platelet count and elevated liver enzymes and billirubin [Table - 2]. Skin biopsy showed features of interface dermatitis. She was treated symptomatically and discharged against medical advice. Patient was lost to follow up.
Discussion
Leflunomide is a new immunomodulatory drug approved by the USFDA in Sept 1998, for the treatment of RA. It inhibits pyrimidine synthesis, resulting in antiproliferative and anti-inflammatory effects. It is a prodrug, which is converted to its active metabolite A771726 with a half-life of two weeks.[1] There are several reports of mild to severe adverse drug reactions associated with leflunomide. The approved/labeled indication for the use of leflunomide is RA.[2],[3] However, it is being recommended for dermatological conditions like psoriatic arthropathy, vasculitis, sarcoidosis and autoimmune bullous diseases.[4]
All five patients reported here had taken leflunomide for RA, 2-6 weeks prior to the onset of symptoms. They presented with fever, skin rash and internal organ involvement. Skin lesions started as exanthematous and purpuric rash, which progressed to erythroderma in three cases and TEN in one case. All patients had long lasting skin lesions. Three patients had lymphadenopathy and three had diarrhea. All patients had leukocytosis; one had eosinophilia; two patients had hyperbilirubinemia. Skin biopsy was done in three cases. Histopathology showed vacuolar degeneration of basal cells, exocytosis, focal subepidermal clefting, occasional necrotic keratinocytes and perivascular lymphocytic infiltration.
Leflunomide was stopped in all patients. Systemic steroids and other supportive treatment were given. Two patients received oral chloestyramine 4 gm tid. Chloestyramine is expected to help in the excretion of leflunomide by interfering with biliary secretion and reabsorption of leflunomide.[5] Three of the five patients recovered completely, one was lost to follow up, while, one died. We could not ascertain the cause of death, as the patient was shifted to another hospital, where she died. A similar case of leflunomide-induced severe cutaneous reaction with hepatitis and skin rashes with fatal outcome, is reported in Indian literature.[5]
All our patients had characteristic pattern of events like delayed onset of reaction, fever, widespread and long lasting skin rash and internal organ involvement. These features suggest a possibility of drug hypersensitivity syndrome (DHS). Other differential diagnosis considered in these cases, were infections such as Epstein Barr virus infection, Kawasaki disease, HIV infection, viral hepatitis, enteric fever, rickettsial infection, graft-versus-host disease, lymphoma and autoimmune connective tissue diseases.
We assessed the causality of reported ADRs by adopting two different causality assessment scales viz. World Health Organization′s ADR probability scale[6] and Naranjo′s scale.[7] Upon assessment of causality by taking all the relevant data into the account, four reactions were found probable and one was possible for leflunomide by both scales. The pathogenesis of DHS is not well understood. These patients show a partially inherited increased susceptibility to the toxic effects of oxidative drug metabolites. These reactive metabolites cause immunological reaction by either forming hapten or by danger signalling. An interaction between drugs and viral infection like HIV has been implicated in the pathogenesis of DHS.[8],[9]
The current recommended dosage schedule for leflunomide is 100 mg once daily for 3 days, followed by 20 mg once daily. This initial high dosage may trigger the formation of toxic metabolites, with resultant clinical syndrome of multiorgan dysfunction. The gradually increased dose regimen as in case of nevirapine and lamotrigine, may lead to adaptive changes that reduce the risk of hypersensitivity reaction in susceptible individuals, making this method of drug administration, a form of prophylactic drug desensitization.[10]
Five cases of severe adverse drug reaction to leflunomide with clinical manifestations suggesting DHS, are reported. We believe that clinicians should be alerted to the possibility of the severe reactions reported here. Careful dosing and periodic monitoring of patients treated with leflunomide for possible adverse drug reaction is recommended.
| 1. |
Olsen NJ, Stein M. New drugs for rheumatoid arthritis. N Engl J Med 2004;350:2167-79.
[Google Scholar]
|
| 2. |
Coblyn JS, Shadick N. Helfgots S. Leflunomide associated weight loss in Rheumatoid Arthritis. Arthritis Rheum 2001;45:1048-51.
[Google Scholar]
|
| 3. |
Koenig AS, Abruzzo JL. Leflunomide induced fevers, thrombocytosis and leukocytosis in a patient with relapsing polychondritis. J Rheumatol 2002;29:192-4.
[Google Scholar]
|
| 4. |
Pudukadan D, Thappa DM. Adverse cutaneous drug reaction; Clinical pattern and causative agents in a tertiary care center in South India. Indian J Dermatol Venereol Leprol. 2004;70:20-4.
[Google Scholar]
|
| 5. |
Uppal M, Roy R, Srinivas CR. Leflunomide induced drug rash and hepatotoxicity. Indian J Dermatol 2004;49:154-5.
[Google Scholar]
|
| 6. |
World Health Organization. Technical Report Series 498. World Health Organization: Geneva; 1972.
[Google Scholar]
|
| 7. |
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al . A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239-45.
[Google Scholar]
|
| 8. |
Sullivan JR, Shear NH. The drug hypersensitivity syndrome: What is the pathogenesis? Arch Dermatol 2001;137:357-64.
[Google Scholar]
|
| 9. |
Sandra RK, Vetrechi J, Shear NH. Idiosyncratic drug reaction; The reactive metabolite syndrome. Lancet 2000;356:1587-91.
[Google Scholar]
|
| 10. |
Wong I, Mewer G, Sander J. Factors influencing the incidence of Lamotrigine -related skin rash. Ann Pharmacother 1999;33:1037-42.
[Google Scholar]
|
Fulltext Views
3,215
PDF downloads
1,292





